[English] 日本語
 Yorodumi
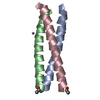
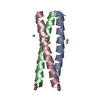
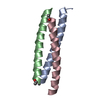
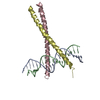
Yorodumi- PDB-2yo0: Salmonella enterica SadA 1049-1304 fused to GCN4 adaptors (SadAK9-cfI) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2yo0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Salmonella enterica SadA 1049-1304 fused to GCN4 adaptors (SadAK9-cfI) | ||||||
 Components Components | GENERAL CONTROL PROTEIN GCN4, PUTATIVE INNER MEMBRANE PROTEIN | ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / HANS MOTIF / YADA-LIKE HEAD / YLHEAD / HEAD INSERT MOTIF / HIM / MEMBRANE PROTEIN / HANS MOTIF / YADA-LIKE HEAD / YLHEAD / HEAD INSERT MOTIF / HIM /  TRIMERIC AUTOTRANSPORTER ADHESIN / TAA / TRIMERIC AUTOTRANSPORTER ADHESIN / TAA /  CHIMERA CHIMERA | ||||||
| Function / homology |  Function and homology information Function and homology informationprotein localization to nuclear periphery / Activation of the AP-1 family of transcription factors / response to amino acid starvation /  mediator complex binding / negative regulation of ribosomal protein gene transcription by RNA polymerase II / positive regulation of cellular response to amino acid starvation / nitrogen catabolite activation of transcription from RNA polymerase II promoter / TFIID-class transcription factor complex binding / amino acid biosynthetic process / positive regulation of transcription initiation by RNA polymerase II ...protein localization to nuclear periphery / Activation of the AP-1 family of transcription factors / response to amino acid starvation / mediator complex binding / negative regulation of ribosomal protein gene transcription by RNA polymerase II / positive regulation of cellular response to amino acid starvation / nitrogen catabolite activation of transcription from RNA polymerase II promoter / TFIID-class transcription factor complex binding / amino acid biosynthetic process / positive regulation of transcription initiation by RNA polymerase II ...protein localization to nuclear periphery / Activation of the AP-1 family of transcription factors / response to amino acid starvation /  mediator complex binding / negative regulation of ribosomal protein gene transcription by RNA polymerase II / positive regulation of cellular response to amino acid starvation / nitrogen catabolite activation of transcription from RNA polymerase II promoter / TFIID-class transcription factor complex binding / amino acid biosynthetic process / positive regulation of transcription initiation by RNA polymerase II / positive regulation of RNA polymerase II transcription preinitiation complex assembly / cellular response to amino acid starvation / cell outer membrane / RNA polymerase II transcription regulator complex / : / mediator complex binding / negative regulation of ribosomal protein gene transcription by RNA polymerase II / positive regulation of cellular response to amino acid starvation / nitrogen catabolite activation of transcription from RNA polymerase II promoter / TFIID-class transcription factor complex binding / amino acid biosynthetic process / positive regulation of transcription initiation by RNA polymerase II / positive regulation of RNA polymerase II transcription preinitiation complex assembly / cellular response to amino acid starvation / cell outer membrane / RNA polymerase II transcription regulator complex / : /  protein transport / DNA-binding transcription activator activity, RNA polymerase II-specific / protein transport / DNA-binding transcription activator activity, RNA polymerase II-specific /  transcription regulator complex / RNA polymerase II-specific DNA-binding transcription factor binding / sequence-specific DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / intracellular signal transduction / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / transcription regulator complex / RNA polymerase II-specific DNA-binding transcription factor binding / sequence-specific DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / intracellular signal transduction / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity /  chromatin binding / negative regulation of transcription by RNA polymerase II / chromatin binding / negative regulation of transcription by RNA polymerase II /  cell surface / positive regulation of transcription by RNA polymerase II / identical protein binding / cell surface / positive regulation of transcription by RNA polymerase II / identical protein binding /  nucleus nucleusSimilarity search - Function | ||||||
| Biological species |   SACCHAROMYCES CEREVISIAE (brewer's yeast) SACCHAROMYCES CEREVISIAE (brewer's yeast)  SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR TYPHIMURIUM (bacteria) SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR TYPHIMURIUM (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | ||||||
 Authors Authors | Hartmann, M.D. / Hernandez Alvarez, B. / Lupas, A.N. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2012 Journal: Proc.Natl.Acad.Sci.USA / Year: 2012Title: Complete Fiber Structures of Complex Trimeric Autotransporter Adhesins Conserved in Enterobacteria. Authors: Hartmann, M.D. / Grin, I. / Dunin-Horkawicz, S. / Deiss, S. / Linke, D. / Lupas, A.N. / Hernandez Alvarez, B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2yo0.cif.gz 2yo0.cif.gz | 64.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2yo0.ent.gz pdb2yo0.ent.gz | 47.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2yo0.json.gz 2yo0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yo/2yo0 https://data.pdbj.org/pub/pdb/validation_reports/yo/2yo0 ftp://data.pdbj.org/pub/pdb/validation_reports/yo/2yo0 ftp://data.pdbj.org/pub/pdb/validation_reports/yo/2yo0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2ynyC  2ynzC  2yo1C  2yo2C  2yo3C  1gcmS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 34005.398 Da / Num. of mol.: 1 Fragment: GCN4 ADAPTOR, RESIDUES 250-278, ADHESIN, RESIDUES 1049-1304 Mutation: YES Source method: isolated from a genetically manipulated source Details: N- AND C-TERMINAL IN-REGISTER FUSION TO GCN4 ADAPTORS Source: (gene. exp.)   SACCHAROMYCES CEREVISIAE (brewer's yeast), (gene. exp.) SACCHAROMYCES CEREVISIAE (brewer's yeast), (gene. exp.)   SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR TYPHIMURIUM (bacteria) SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR TYPHIMURIUM (bacteria)Production host:   ESCHERICHIA COLI (E. coli) / References: UniProt: P03069, UniProt: Q8ZL64 ESCHERICHIA COLI (E. coli) / References: UniProt: P03069, UniProt: Q8ZL64 |
|---|---|
| #2: Chemical | ChemComp-CL /  Chloride Chloride |
| #3: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.6 Å3/Da / Density % sol: 66 % / Description: NONE |
|---|---|
Crystal grow | Details: 20% (V/V) BUTANEDIOL, 100 MM SODIUM ACETATE PH 4.5 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 1 / Beamline: X10SA / Wavelength: 1 | |||||||||||||||
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Sep 7, 2008 | |||||||||||||||
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 | |||||||||||||||
| Reflection twin |
| |||||||||||||||
| Reflection | Resolution: 2.8→37.2 Å / Num. obs: 12334 / % possible obs: 99.5 % / Redundancy: 3.25 % / Rmerge(I) obs: 0.14 / Net I/σ(I): 8.59 | |||||||||||||||
| Reflection shell | Resolution: 2.8→2.97 Å / Redundancy: 3.22 % / Rmerge(I) obs: 0.6 / Mean I/σ(I) obs: 2.07 / % possible all: 99.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1GCM Resolution: 2.8→34.53 Å / Cor.coef. Fo:Fc: 0.9 / Cor.coef. Fo:Fc free: 0.781 / SU B: 8.128 / SU ML: 0.174 / Cross valid method: THROUGHOUT / ESU R: 0.097 / ESU R Free: 0.074 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34.145 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→34.53 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller








































































 PDBj
PDBj



