+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2r2l | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of Farnesyl Protein Transferase bound to PB-93 | ||||||
 Components Components | (Farnesyltransferase subunit ... ) x 2 ) x 2 | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  Malaria / FPT / Metal-binding / Malaria / FPT / Metal-binding /  Phosphorylation / Phosphorylation /  Prenyltransferase / Prenyltransferase /  Zinc Zinc | ||||||
| Function / homology |  Function and homology information Function and homology informationskeletal muscle acetylcholine-gated channel clustering / Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex /  protein geranylgeranyltransferase type I / protein farnesylation / regulation of fibroblast proliferation ...skeletal muscle acetylcholine-gated channel clustering / Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein geranylgeranyltransferase type I / protein farnesylation / regulation of fibroblast proliferation ...skeletal muscle acetylcholine-gated channel clustering / Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex /  protein geranylgeranyltransferase type I / protein farnesylation / regulation of fibroblast proliferation / positive regulation of tubulin deacetylation / protein geranylgeranyltransferase type I / protein farnesylation / regulation of fibroblast proliferation / positive regulation of tubulin deacetylation /  protein farnesyltransferase / protein farnesyltransferase /  protein farnesyltransferase activity / protein farnesyltransferase activity /  protein farnesyltransferase complex / protein farnesyltransferase complex /  Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering / Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering /  farnesyltranstransferase activity / negative regulation of nitric-oxide synthase biosynthetic process / microtubule associated complex / farnesyltranstransferase activity / negative regulation of nitric-oxide synthase biosynthetic process / microtubule associated complex /  enzyme-linked receptor protein signaling pathway / positive regulation of nitric-oxide synthase biosynthetic process / positive regulation of Rac protein signal transduction / alpha-tubulin binding / response to inorganic substance / positive regulation of cell cycle / response to cytokine / enzyme-linked receptor protein signaling pathway / positive regulation of nitric-oxide synthase biosynthetic process / positive regulation of Rac protein signal transduction / alpha-tubulin binding / response to inorganic substance / positive regulation of cell cycle / response to cytokine /  wound healing / lipid metabolic process / response to organic cyclic compound / wound healing / lipid metabolic process / response to organic cyclic compound /  receptor tyrosine kinase binding / positive regulation of fibroblast proliferation / fibroblast proliferation / receptor tyrosine kinase binding / positive regulation of fibroblast proliferation / fibroblast proliferation /  microtubule binding / cell population proliferation / molecular adaptor activity / negative regulation of cell population proliferation / positive regulation of cell population proliferation / negative regulation of apoptotic process / zinc ion binding / microtubule binding / cell population proliferation / molecular adaptor activity / negative regulation of cell population proliferation / positive regulation of cell population proliferation / negative regulation of apoptotic process / zinc ion binding /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.23 Å X-RAY DIFFRACTION / Resolution: 2.23 Å | ||||||
 Authors Authors | Strickland, C.O. / Voorhis, W. | ||||||
 Citation Citation |  Journal: Antimicrob.Agents Chemother. / Year: 2007 Journal: Antimicrob.Agents Chemother. / Year: 2007Title: Efficacy, pharmacokinetics, and metabolism of tetrahydroquinoline inhibitors of Plasmodium falciparum protein farnesyltransferase. Authors: Van Voorhis, W.C. / Rivas, K.L. / Bendale, P. / Nallan, L. / Horney, C. / Barrett, L.K. / Bauer, K.D. / Smart, B.P. / Ankala, S. / Hucke, O. / Verlinde, C.L. / Chakrabarti, D. / Strickland, ...Authors: Van Voorhis, W.C. / Rivas, K.L. / Bendale, P. / Nallan, L. / Horney, C. / Barrett, L.K. / Bauer, K.D. / Smart, B.P. / Ankala, S. / Hucke, O. / Verlinde, C.L. / Chakrabarti, D. / Strickland, C. / Yokoyama, K. / Buckner, F.S. / Hamilton, A.D. / Williams, D.K. / Lombardo, L.J. / Floyd, D. / Gelb, M.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2r2l.cif.gz 2r2l.cif.gz | 172.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2r2l.ent.gz pdb2r2l.ent.gz | 133.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2r2l.json.gz 2r2l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/r2/2r2l https://data.pdbj.org/pub/pdb/validation_reports/r2/2r2l ftp://data.pdbj.org/pub/pdb/validation_reports/r2/2r2l ftp://data.pdbj.org/pub/pdb/validation_reports/r2/2r2l | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Farnesyltransferase subunit ... , 2 types, 2 molecules AB
| #1: Protein |  Mass: 37916.566 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Fnta / Production host: Rattus norvegicus (Norway rat) / Gene: Fnta / Production host:   Escherichia coli (E. coli) / References: UniProt: Q5RKJ4, UniProt: Q04631*PLUS Escherichia coli (E. coli) / References: UniProt: Q5RKJ4, UniProt: Q04631*PLUS |
|---|---|
| #2: Protein |  / CAAX farnesyltransferase subunit beta / RAS proteins prenyltransferase beta / FTase-beta / CAAX farnesyltransferase subunit beta / RAS proteins prenyltransferase beta / FTase-betaMass: 44879.297 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Fntb / Production host: Rattus norvegicus (Norway rat) / Gene: Fntb / Production host:   Escherichia coli (E. coli) / References: UniProt: Q02293, Escherichia coli (E. coli) / References: UniProt: Q02293,  protein farnesyltransferase protein farnesyltransferase |
-Non-polymers , 4 types, 537 molecules 




| #3: Chemical | ChemComp-ZN / |
|---|---|
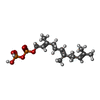
| #4: Chemical | ChemComp-FPP /  Farnesyl pyrophosphate Farnesyl pyrophosphate |
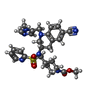
| #5: Chemical | ChemComp-PB9 / |
| #6: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.55 Å3/Da / Density % sol: 65.36 % |
|---|---|
Crystal grow | Method: vapor diffusion, hanging drop / Details: VAPOR DIFFUSION, HANGING DROP |
-Data collection
| Diffraction | Mean temperature: 95 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS HTC / Detector: IMAGE PLATE / Details: Max Flux |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.23→20 Å / Num. all: 56752 / Num. obs: 56633 / % possible obs: 100 % / Observed criterion σ(F): -3 / Observed criterion σ(I): -3 / Redundancy: 4.2 % / Biso Wilson estimate: 41.3 Å2 / Rmerge(I) obs: 0.061 / Rsym value: 0.061 / Χ2: 1 / Net I/σ(I): 17.3 |
| Reflection shell | Resolution: 2.23→2.27 Å / Redundancy: 3.5 % / Rmerge(I) obs: 0.308 / Mean I/σ(I) obs: 4.2 / Num. unique all: 2780 / Rsym value: 0.308 / Χ2: 1 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.23→20 Å / FOM work R set: 0.856
| ||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 42.366 Å2 | ||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.23→20 Å
| ||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj




