+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2jk7 | ||||||
|---|---|---|---|---|---|---|---|
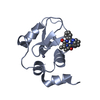
| Title | XIAP BIR3 bound to a Smac Mimetic | ||||||
 Components Components | BACULOVIRAL IAP REPEAT-CONTAINING PROTEIN 4 | ||||||
 Keywords Keywords |  APOPTOSIS / APOPTOSIS /  ZINC-FINGER / POLYMORPHISM / ZINC-FINGER / POLYMORPHISM /  SMAC MIMETIC / METAL-BINDING / BIR3 / SMAC MIMETIC / METAL-BINDING / BIR3 /  ZINC / ZINC /  LIGASE / LIGASE /  CYTOPLASM / X-LINKED INHIBITOR OF APOPTOSIS / UBL CONJUGATION PATHWAY / CYTOPLASM / X-LINKED INHIBITOR OF APOPTOSIS / UBL CONJUGATION PATHWAY /  THIOL PROTEASE INHIBITOR / THIOL PROTEASE INHIBITOR /  PHOSPHOPROTEIN / UBL CONJUGATION / PHOSPHOPROTEIN / UBL CONJUGATION /  PROTEASE INHIBITOR PROTEASE INHIBITOR | ||||||
| Function / homology |  Function and homology information Function and homology informationendopeptidase regulator activity / regulation of apoptosis involved in tissue homeostasis / inhibition of cysteine-type endopeptidase activity / positive regulation of protein linear polyubiquitination / regulation of BMP signaling pathway / copper ion homeostasis / nucleotide-binding oligomerization domain containing 1 signaling pathway / regulation of nucleotide-binding domain, leucine rich repeat containing receptor signaling pathway / protein serine/threonine kinase binding / nucleotide-binding oligomerization domain containing 2 signaling pathway ...endopeptidase regulator activity / regulation of apoptosis involved in tissue homeostasis / inhibition of cysteine-type endopeptidase activity / positive regulation of protein linear polyubiquitination / regulation of BMP signaling pathway / copper ion homeostasis / nucleotide-binding oligomerization domain containing 1 signaling pathway / regulation of nucleotide-binding domain, leucine rich repeat containing receptor signaling pathway / protein serine/threonine kinase binding / nucleotide-binding oligomerization domain containing 2 signaling pathway / SMAC, XIAP-regulated apoptotic response / Regulation of the apoptosome activity / Activation of caspases through apoptosome-mediated cleavage / SMAC (DIABLO) binds to IAPs / SMAC(DIABLO)-mediated dissociation of IAP:caspase complexes / TNFR1-induced proapoptotic signaling / RIPK1-mediated regulated necrosis / cysteine-type endopeptidase inhibitor activity /  regulation of innate immune response / protein K63-linked ubiquitination / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / positive regulation of type I interferon production / negative regulation of tumor necrosis factor-mediated signaling pathway / Regulation of PTEN localization / TNFR1-induced NF-kappa-B signaling pathway / positive regulation of protein ubiquitination / Deactivation of the beta-catenin transactivating complex / Regulation of TNFR1 signaling / positive regulation of JNK cascade / RING-type E3 ubiquitin transferase / negative regulation of cysteine-type endopeptidase activity involved in apoptotic process / Regulation of necroptotic cell death / regulation of innate immune response / protein K63-linked ubiquitination / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / positive regulation of type I interferon production / negative regulation of tumor necrosis factor-mediated signaling pathway / Regulation of PTEN localization / TNFR1-induced NF-kappa-B signaling pathway / positive regulation of protein ubiquitination / Deactivation of the beta-catenin transactivating complex / Regulation of TNFR1 signaling / positive regulation of JNK cascade / RING-type E3 ubiquitin transferase / negative regulation of cysteine-type endopeptidase activity involved in apoptotic process / Regulation of necroptotic cell death /  Wnt signaling pathway / Regulation of PTEN stability and activity / ubiquitin-protein transferase activity / positive regulation of canonical Wnt signaling pathway / Wnt signaling pathway / Regulation of PTEN stability and activity / ubiquitin-protein transferase activity / positive regulation of canonical Wnt signaling pathway /  ubiquitin protein ligase activity / regulation of cell population proliferation / ubiquitin protein ligase activity / regulation of cell population proliferation /  regulation of inflammatory response / neuron apoptotic process / regulation of apoptotic process / positive regulation of canonical NF-kappaB signal transduction / regulation of inflammatory response / neuron apoptotic process / regulation of apoptotic process / positive regulation of canonical NF-kappaB signal transduction /  regulation of cell cycle / defense response to bacterium / DNA damage response / negative regulation of apoptotic process / regulation of cell cycle / defense response to bacterium / DNA damage response / negative regulation of apoptotic process /  nucleoplasm / identical protein binding / nucleoplasm / identical protein binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.82 Å MOLECULAR REPLACEMENT / Resolution: 2.82 Å | ||||||
 Authors Authors | Saito, N.G. / Meagher, J.L. / Stuckey, J.A. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2008 Journal: J.Med.Chem. / Year: 2008Title: Structure-Based Design, Synthesis, Evaluation, and Crystallographic Studies of Conformationally Constrained Smac Mimetics as Inhibitors of the X-Linked Inhibitor of Apoptosis Protein (Xiap). Authors: Sun, H. / Stuckey, J.A. / Nikolovska-Coleska, Z. / Qin, D. / Meagher, J.L. / Qiu, S. / Lu, J. / Yang, C. / Saito, N.G. / Wang, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2jk7.cif.gz 2jk7.cif.gz | 36.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2jk7.ent.gz pdb2jk7.ent.gz | 23.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2jk7.json.gz 2jk7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jk/2jk7 https://data.pdbj.org/pub/pdb/validation_reports/jk/2jk7 ftp://data.pdbj.org/pub/pdb/validation_reports/jk/2jk7 ftp://data.pdbj.org/pub/pdb/validation_reports/jk/2jk7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 13293.755 Da / Num. of mol.: 1 / Fragment: BIR3, RESIDUES 241-356 Source method: isolated from a genetically manipulated source Details: SMALL MOLECULE SMAC MIMETIC / Source: (gene. exp.)   HOMO SAPIENS (human) / Plasmid: PET28 / Production host: HOMO SAPIENS (human) / Plasmid: PET28 / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P98170 ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P98170 |
|---|---|
| #2: Chemical | ChemComp-BI6 / ( |
| #3: Chemical | ChemComp-ZN / |
| #4: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.6 Å3/Da / Density % sol: 66 % / Description: NONE |
|---|---|
Crystal grow | pH: 7.5 Details: 20% POLYETHYLENE GLYCOL 8000 0.2M MGCL2 0.1M TRIS, PH 8.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 32-ID / Wavelength: 1 / Beamline: 32-ID / Wavelength: 1 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Jul 14, 2004 / Details: DIAMOND |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→50 Å / Num. obs: 6181 / % possible obs: 99.3 % / Observed criterion σ(I): 2 / Redundancy: 9 % / Biso Wilson estimate: 61.9 Å2 / Rmerge(I) obs: 0.07 / Net I/σ(I): 20 |
| Reflection shell | Resolution: 2.8→2.9 Å / Redundancy: 9 % / Rmerge(I) obs: 0.3 / Mean I/σ(I) obs: 3 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: UNPUBLISHED STRUCTURE IN LAB Resolution: 2.82→6 Å / Rfactor Rfree error: 0.012 / Data cutoff high absF: 414409.45 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 73.6531 Å2 / ksol: 0.467133 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 60.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.82→6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.8→2.96 Å / Rfactor Rfree error: 0.058 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller
























 PDBj
PDBj













