[English] 日本語
 Yorodumi
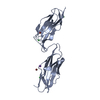
Yorodumi- PDB-1vca: CRYSTAL STRUCTURE OF AN INTEGRIN-BINDING FRAGMENT OF VASCULAR CEL... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1vca | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF AN INTEGRIN-BINDING FRAGMENT OF VASCULAR CELL ADHESION MOLECULE-1 AT 1.8 ANGSTROMS RESOLUTION | ||||||
 Components Components | HUMAN VASCULAR CELL ADHESION MOLECULE-1 VCAM-1 VCAM-1 | ||||||
 Keywords Keywords |  CELL ADHESION PROTEIN / CELL ADHESION PROTEIN /  IMMUNOGLOBULIN SUPERFAMILY / INTEGRIN-BINDING IMMUNOGLOBULIN SUPERFAMILY / INTEGRIN-BINDING | ||||||
| Function / homology |  Function and homology information Function and homology informationcardiac neuron differentiation / alpha9-beta1 integrin-vascular cell adhesion molecule-1 complex / cell adhesion mediator activity /  cell-cell adhesion in response to extracellular stimulus / chronic inflammatory response / membrane to membrane docking / cell-cell adhesion in response to extracellular stimulus / chronic inflammatory response / membrane to membrane docking /  primary amine oxidase activity / leukocyte tethering or rolling / primary amine oxidase activity / leukocyte tethering or rolling /  innervation / amine metabolic process ...cardiac neuron differentiation / alpha9-beta1 integrin-vascular cell adhesion molecule-1 complex / cell adhesion mediator activity / innervation / amine metabolic process ...cardiac neuron differentiation / alpha9-beta1 integrin-vascular cell adhesion molecule-1 complex / cell adhesion mediator activity /  cell-cell adhesion in response to extracellular stimulus / chronic inflammatory response / membrane to membrane docking / cell-cell adhesion in response to extracellular stimulus / chronic inflammatory response / membrane to membrane docking /  primary amine oxidase activity / leukocyte tethering or rolling / primary amine oxidase activity / leukocyte tethering or rolling /  innervation / amine metabolic process / innervation / amine metabolic process /  podosome / heterotypic cell-cell adhesion / heterophilic cell-cell adhesion via plasma membrane cell adhesion molecules / leukocyte cell-cell adhesion / response to ionizing radiation / response to zinc ion / podosome / heterotypic cell-cell adhesion / heterophilic cell-cell adhesion via plasma membrane cell adhesion molecules / leukocyte cell-cell adhesion / response to ionizing radiation / response to zinc ion /  microvillus / microvillus /  : / cellular response to vascular endothelial growth factor stimulus / Integrin cell surface interactions / positive regulation of T cell proliferation / : / cellular response to vascular endothelial growth factor stimulus / Integrin cell surface interactions / positive regulation of T cell proliferation /  cell adhesion molecule binding / cell chemotaxis / response to nutrient / cell-matrix adhesion / B cell differentiation / response to nicotine / cell adhesion molecule binding / cell chemotaxis / response to nutrient / cell-matrix adhesion / B cell differentiation / response to nicotine /  filopodium / filopodium /  sarcolemma / cellular response to amyloid-beta / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / Interferon gamma signaling / sarcolemma / cellular response to amyloid-beta / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / Interferon gamma signaling /  integrin binding / apical part of cell / cellular response to tumor necrosis factor / response to ethanol / Interleukin-4 and Interleukin-13 signaling / response to lipopolysaccharide / response to hypoxia / integrin binding / apical part of cell / cellular response to tumor necrosis factor / response to ethanol / Interleukin-4 and Interleukin-13 signaling / response to lipopolysaccharide / response to hypoxia /  early endosome / early endosome /  cell adhesion / cell adhesion /  inflammatory response / external side of plasma membrane / inflammatory response / external side of plasma membrane /  Golgi apparatus / Golgi apparatus /  cell surface / cell surface /  endoplasmic reticulum / endoplasmic reticulum /  extracellular space / extracellular exosome / extracellular space / extracellular exosome /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.8 Å SYNCHROTRON / Resolution: 1.8 Å | ||||||
 Authors Authors | Jones, E.Y. / Harlos, K. / Bottomley, M.J. / Robinson, R.C. / Driscoll, P.C. / Edwards, R.M. / Clements, J.M. / Dudgeon, T.J. / Stuart, D.I. | ||||||
 Citation Citation |  Journal: Nature / Year: 1995 Journal: Nature / Year: 1995Title: Crystal structure of an integrin-binding fragment of vascular cell adhesion molecule-1 at 1.8 A resolution. Authors: Jones, E.Y. / Harlos, K. / Bottomley, M.J. / Robinson, R.C. / Driscoll, P.C. / Edwards, R.M. / Clements, J.M. / Dudgeon, T.J. / Stuart, D.I. #1:  Journal: J.Mol.Biol. / Year: 1994 Journal: J.Mol.Biol. / Year: 1994Title: Crystallization and Preliminary X-Ray Diffraction Characterisation of Both a Native and Selenomethionyl Vla-4 Binding Fragment of Vcam-1 Authors: Bottomley, M.J. / Robinson, R.C. / Driscoll, P.C. / Harlos, K. / Stuart, D.I. / Aplin, R.T. / Clements, J.M. / Jones, E.Y. / Dudgeon, T.J. #2:  Journal: Eur.J.Biochem. / Year: 1994 Journal: Eur.J.Biochem. / Year: 1994Title: Expression and Characterisation of a Very-Late Antigen-4 (Alpha4Beta1) Integrin Binding Fragment of Vascular Cell-Adhesion Molecule-1 Authors: Dudgeon, T.J. / Bottomley, M.J. / Driscoll, P.C. / Humphries, M.J. / Mould, A.P. / Wingfield, G.I. / Clements, J.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1vca.cif.gz 1vca.cif.gz | 89.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1vca.ent.gz pdb1vca.ent.gz | 73 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1vca.json.gz 1vca.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vc/1vca https://data.pdbj.org/pub/pdb/validation_reports/vc/1vca ftp://data.pdbj.org/pub/pdb/validation_reports/vc/1vca ftp://data.pdbj.org/pub/pdb/validation_reports/vc/1vca | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO A 7 / 2: CIS PROLINE - PRO A 60 / 3: CIS PROLINE - PRO A 120 / 4: CIS PROLINE - PRO B 7 / 5: CIS PROLINE - PRO B 60 / 6: CIS PROLINE - PRO B 120 |
- Components
Components
| #1: Protein |  VCAM-1 / VCAM-D1 / 2 VCAM-1 / VCAM-D1 / 2Mass: 22535.574 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Strain: HW1110 / Production host: Homo sapiens (human) / Strain: HW1110 / Production host:   Escherichia coli (E. coli) / References: UniProt: P19320 Escherichia coli (E. coli) / References: UniProt: P19320#2: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 44.07 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | *PLUS Method: vapor diffusion, sitting drop / Details: Bottomley, M.J., (1994) J.Mol.Biol., 244, 464. | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Photon Factory Photon Factory  / Beamline: BL-6A / Wavelength: 0.97 Å / Beamline: BL-6A / Wavelength: 0.97 Å |
|---|---|
| Detector | Type: FUJI / Detector: IMAGE PLATE / Date: 1994 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97 Å / Relative weight: 1 : 0.97 Å / Relative weight: 1 |
| Reflection | Num. obs: 32077 / % possible obs: 85 % / Observed criterion σ(I): 0 / Redundancy: 4.5 % / Rmerge(I) obs: 0.067 |
| Reflection | *PLUS Highest resolution: 1.8 Å / Rmerge(I) obs: 0.087 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.8→15 Å / σ(F): 0 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→15 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj










