[English] 日本語
 Yorodumi
Yorodumi- PDB-1gp6: Anthocyanidin synthase from Arabidopsis thaliana complexed with t... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gp6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Anthocyanidin synthase from Arabidopsis thaliana complexed with trans-dihydroquercetin (with 30 min exposure to O2) | ||||||
 Components Components | LEUCOANTHOCYANIDIN DIOXYGENASE Leucocyanidin oxygenase Leucocyanidin oxygenase | ||||||
 Keywords Keywords |  OXIDOREDUCTASE / OXIDOREDUCTASE /  OXYGENASE / 2-OXOGLUTARATE DEPENDENT DIOXYGENASE / OXYGENASE / 2-OXOGLUTARATE DEPENDENT DIOXYGENASE /  FLAVONOID BIOSYNTHESIS FLAVONOID BIOSYNTHESIS | ||||||
| Function / homology |  Function and homology information Function and homology information anthocyanidin synthase / proanthocyanidin biosynthetic process / anthocyanidin synthase / proanthocyanidin biosynthetic process /  leucocyanidin oxygenase activity / anthocyanin-containing compound biosynthetic process / response to jasmonic acid / vacuole organization / leucocyanidin oxygenase activity / anthocyanin-containing compound biosynthetic process / response to jasmonic acid / vacuole organization /  L-ascorbic acid binding / response to wounding / L-ascorbic acid binding / response to wounding /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |   ARABIDOPSIS THALIANA (thale cress) ARABIDOPSIS THALIANA (thale cress) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.75 Å MOLECULAR REPLACEMENT / Resolution: 1.75 Å | ||||||
 Authors Authors | Wilmouth, R.C. / Turnbull, J.J. / Welford, R.W.D. / Clifton, I.J. / Prescott, A.G. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Structure / Year: 2002 Journal: Structure / Year: 2002Title: Structure and Mechanism of Anthocyanidin Synthase from Arabidopsis Thaliana. Authors: Wilmouth, R.C. / Turnbull, J.J. / Welford, R.W.D. / Clifton, I.J. / Prescott, A.G. / Schofield, C.J. #1:  Journal: Chem.Commun. / Year: 2000 Journal: Chem.Commun. / Year: 2000Title: Are Anthocyanidins the Immediate Products of Anthocyanidin Synthase? Authors: Turnbull, J.J. / Sobey, W.J. / Aplin, R.T. / Hassan, A. / Firmin, J.L. / Schofield, C.J. / Prescott, A.G. #2: Journal: Acta Crystallogr.,Sect.D / Year: 2001 Title: Purification, Crystallization and Preliminary X-Ray Diffraction of Anthocyanidin Synthase from Arabidopsis Thaliana Authors: Turnbull, J.J. / Prescott, A.G. / Schofield, C.J. / Wilmouth, R.C. #3: Journal: Plant J. / Year: 1999 Title: Direct Evidence for Anthocyanidin Synthase as a 2-Oxoglutarate-Dependent Oxygenase: Molecular Cloning and Functional Expression of Cdna from a Red Forma of Perilla Frutescens Authors: Saito, K. / Kobayashi, M. / Gong, Z. / Tanaka, Y. / Yamazaki, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gp6.cif.gz 1gp6.cif.gz | 94.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gp6.ent.gz pdb1gp6.ent.gz | 69.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gp6.json.gz 1gp6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gp/1gp6 https://data.pdbj.org/pub/pdb/validation_reports/gp/1gp6 ftp://data.pdbj.org/pub/pdb/validation_reports/gp/1gp6 ftp://data.pdbj.org/pub/pdb/validation_reports/gp/1gp6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1gp4SC  1gp5C S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein |  Leucocyanidin oxygenase / LDOX / LEUCOCYANIDIN OXYGENASE / ANTHOCYANIDIN SYNTHASE / ANS / LEUCOANTHOCYANIDIN HYDROXYLASE / ...LDOX / LEUCOCYANIDIN OXYGENASE / ANTHOCYANIDIN SYNTHASE / ANS / LEUCOANTHOCYANIDIN HYDROXYLASE / PROTEIN TANNIN DEFICIENT SEED 4 / TDS4 / PROTEIN TRANSPARENT TESTA 11 / TT11 / PROTEIN TRANSPARENT TESTA 17 / TT17 / PROTEIN TRANSPARENT TESTA 18 / TT18 Leucocyanidin oxygenase / LDOX / LEUCOCYANIDIN OXYGENASE / ANTHOCYANIDIN SYNTHASE / ANS / LEUCOANTHOCYANIDIN HYDROXYLASE / ...LDOX / LEUCOCYANIDIN OXYGENASE / ANTHOCYANIDIN SYNTHASE / ANS / LEUCOANTHOCYANIDIN HYDROXYLASE / PROTEIN TANNIN DEFICIENT SEED 4 / TDS4 / PROTEIN TRANSPARENT TESTA 11 / TT11 / PROTEIN TRANSPARENT TESTA 17 / TT17 / PROTEIN TRANSPARENT TESTA 18 / TT18Mass: 40451.352 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   ARABIDOPSIS THALIANA (thale cress) / Plasmid: PET-24A / Production host: ARABIDOPSIS THALIANA (thale cress) / Plasmid: PET-24A / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q96323, EC: 1.14.11.19 ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q96323, EC: 1.14.11.19 |
|---|
-Non-polymers , 6 types, 363 molecules 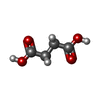








| #2: Chemical | ChemComp-SIN /  Succinic acid Succinic acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|
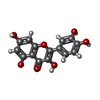
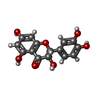
| #3: Chemical |  MES (buffer) MES (buffer)#4: Chemical | ChemComp-QUE / |  Quercetin Quercetin#5: Chemical | ChemComp-DH2 / ( | #6: Chemical | ChemComp-FE2 / | #7: Water | ChemComp-HOH / |  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.39 Å3/Da / Density % sol: 48.1 % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | pH: 6.5 Details: 18% (W/V) PEG 2000 MONOMETHYLETHER, 50 MM MES, 200 MM AMMONIUM ACETATE, 2 MM IRON(II) SULPHATE, 10 MM POTASSIUM ALPHA-KETOGLUTARATE, 10 MM SODIUM ASCORBATE 10 MM DHQ (IN MEOH TO GIVE A FINAL ...Details: 18% (W/V) PEG 2000 MONOMETHYLETHER, 50 MM MES, 200 MM AMMONIUM ACETATE, 2 MM IRON(II) SULPHATE, 10 MM POTASSIUM ALPHA-KETOGLUTARATE, 10 MM SODIUM ASCORBATE 10 MM DHQ (IN MEOH TO GIVE A FINAL CONC. OF 10%(V/V) MEOH), PH 6.5, ANAEROBIC (AR ATMOSPHERE, < 0.5 PPM OXYGEN) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging dropDetails: Turnbull, J.J., (2001) Acta Crystallogr.,Sect.D, D57, 425. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SRS SRS  / Beamline: PX9.6 / Wavelength: 0.978 / Beamline: PX9.6 / Wavelength: 0.978 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: May 15, 2001 / Details: MIRRORS |
| Radiation | Monochromator: SI / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.978 Å / Relative weight: 1 : 0.978 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→25 Å / Num. obs: 39711 / % possible obs: 99.9 % / Redundancy: 3.8 % / Biso Wilson estimate: 20 Å2 / Rmerge(I) obs: 0.068 / Net I/σ(I): 14.9 |
| Reflection shell | Resolution: 1.75→1.8 Å / Redundancy: 3.8 % / Rmerge(I) obs: 0.274 / Mean I/σ(I) obs: 4.1 / % possible all: 99.9 |
| Reflection | *PLUS Num. measured all: 335364 |
| Reflection shell | *PLUS Lowest resolution: 1.82 Å / % possible obs: 99.9 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1GP4 Resolution: 1.75→25 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 1528058.88 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 45.2 Å2 / ksol: 0.336 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.75→25 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.75→1.86 Å / Rfactor Rfree error: 0.019 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 1.1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor obs: 0.244 |
 Movie
Movie Controller
Controller












 PDBj
PDBj










