+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1dzo | ||||||
|---|---|---|---|---|---|---|---|
| Title | Truncated PAK pilin from Pseudomonas aeruginosa | ||||||
 Components Components | TYPE IV PILIN | ||||||
 Keywords Keywords |  CELL ADHESION / CELL ADHESION /  LECTIN / ADHESIN LECTIN / ADHESIN | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |   PSEUDOMONAS AERUGINOSA PAK (bacteria) PSEUDOMONAS AERUGINOSA PAK (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MIRAS / Resolution: 1.63 Å MIRAS / Resolution: 1.63 Å | ||||||
 Authors Authors | Hazes, B. / Read, R.J. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: Crystal Structure of Pseudomonas Aeruginosa Pak Pilin Suggests a Main-Chain-Dominated Mode of Receptor Binding Authors: Hazes, B. / Sastry, P.A. / Hayakawa, K. / Read, R.J. / Irvin, R.T. | ||||||
| History |
| ||||||
| Remark 650 | HELIX DETERMINATION METHOD: DSSP |
- Structure visualization
Structure visualization
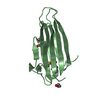
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1dzo.cif.gz 1dzo.cif.gz | 57.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1dzo.ent.gz pdb1dzo.ent.gz | 45.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1dzo.json.gz 1dzo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dz/1dzo https://data.pdbj.org/pub/pdb/validation_reports/dz/1dzo ftp://data.pdbj.org/pub/pdb/validation_reports/dz/1dzo ftp://data.pdbj.org/pub/pdb/validation_reports/dz/1dzo | HTTPS FTP |
|---|
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | ON CELLS PILIN IS FOUND AS LONG THIN FIBERS WHICH MEDIATE CELL ATTACHMENT. BASED ON MOLECULAR MODELING A PRELIMINARY FIBER MODEL HAS BEEN PROPOSED FOR THE RELATED TYPE IV PILIN OF NEISSERIA GONORRHOEAE(PDB ID CODE 1AY2). TO GENERATE THE CORRESPONDING MODEL FOR PAK PILIN THE COORDINATES IN THIS ENTRY SHOULD BE SUPERIMPOSED ON THE NEISSERIA MODEL FOLLOWED BY THE APPLICATION OF THE TRANSFORMATIONS AS INDICATED IN PDB ENTRY 1AY2.PDB ALTHOUGH THE NEISSERIA MODEL IS THE BEST CURRENT MODEL FOR THE FIBER STRUCTURE, IT SHOULD BE KEPT IN MIND THAT SIGNIFICANT DEVIATIONS FROM REALITY MAY EXIST. IN PARTICULAR, IT MAY BE POSSIBLE TO CREATE A SIMILAR MODEL BY STACKING PERFECT PENTAMERS OF PILIN MOLECULES. THE TYPE IV PILUS IS POLAR AND IT APPEARS TO EXPOSE EXTREMELY HYDROPHOBIC ALPHA HELICES AT ONE OF ITS ENDS. BASED ON RECEPTOR BINDING CONSIDERATIONS WE HAVE PROPOSED THAT THE HYDROPHOBIC ALPHA HELICES ARE DISPLAYED AT THE TIP OF THE PILUS AND THEREFORE INTERACT WITH HOST CELLS. THIS CONTRASTS WITH EARLIER MODELS WHERE THE HELICES WERE ASSUMED TO BE BURIED IN THE BACTERIAL OUTER MEMBRANE. |
- Components
Components
| #1: Protein | Mass: 12696.225 Da / Num. of mol.: 1 / Fragment: GLOBULAR DOMAIN / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   PSEUDOMONAS AERUGINOSA PAK (bacteria) PSEUDOMONAS AERUGINOSA PAK (bacteria)Description: RESIDUES 22-28 ARE FROM THE EXPRESSION VECTOR. RESIDUES 29-144 ARE FROM THE MATURE PROTEIN. Cellular location: EXTRACELLULAR FILAMENTOUS APPENDAGE / Gene: PILA / Plasmid: PRLD / Cellular location (production host): PERIPLASMIC SPACE / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21 / References: UniProt: P02973 ESCHERICHIA COLI (E. coli) / Strain (production host): BL21 / References: UniProt: P02973 |
|---|---|
| #2: Water | ChemComp-HOH /  Water Water |
| Compound details | CHAIN A IS A DELETION MUTANT, MISSING RESIDUES 1-28 OF THE NATIVE SEQUENCE. THE RECOMBINANT PROTEIN ...CHAIN A IS A DELETION MUTANT, MISSING RESIDUES 1-28 OF THE NATIVE SEQUENCE. THE RECOMBINAN |
| Sequence details | RESIDUES 22-28 ARE FROM THE EXPRESSION VECTOR. RESIDUES 22-24 HAVE NOT BEEN MODELED DUE TO LACK OF ...RESIDUES 22-28 ARE FROM THE EXPRESSION |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.14 Å3/Da / Density % sol: 43 % Description: DERIVATIVE DATA WERE SCALED USING THE NATIVE DATA AS A REFERENCE | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | Method: vapor diffusion, hanging drop / pH: 8.2 Details: HANGING DROP USING 1 ML OF RESERVOIR DROPS MADE FROM 3 MICROLITRE PROTEIN AND 3 MICROLITRE OF MOTHER LIQUOR PROTEIN SOLUTION = 10 MG/ML IN WATER MOTHER LIQUOR = 60% (NH4)2SO4, 0.1M HEPES PH 8.2 | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: unknown | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 293 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: ELLIOTT GX-13 / Wavelength: 1.5418 ROTATING ANODE / Type: ELLIOTT GX-13 / Wavelength: 1.5418 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Dec 15, 2000 / Details: SUPPER MIRROR |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.633→37.44 Å / Num. obs: 253714 / % possible obs: 99.5 % / Redundancy: 7.6 % / Biso Wilson estimate: 16.8 Å2 / Rsym value: 0.049 / Net I/σ(I): 26.7 |
| Reflection shell | Resolution: 1.63→1.72 Å / Redundancy: 6.6 % / Mean I/σ(I) obs: 10.1 / Rsym value: 0.19 / % possible all: 96.4 |
| Reflection | *PLUS Num. obs: 14500 / Num. measured all: 110628 / Rmerge(I) obs: 0.049 |
| Reflection shell | *PLUS % possible obs: 96.4 % / Rmerge(I) obs: 0.19 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MIRAS / Resolution: 1.63→37.44 Å / SU B: 1.26 / SU ML: 0.04 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.12 / ESU R Free: 0.08 MIRAS / Resolution: 1.63→37.44 Å / SU B: 1.26 / SU ML: 0.04 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.12 / ESU R Free: 0.08 Details: CNS EXPLICIT BULK SOLVENT CORRECTION WAS USED. B-SPHERE RMS = 1.851 FOR FREE ATOMS AND 2.429 FOR BONDED ATOMS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.3 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.63→37.44 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj

