[English] 日本語
 Yorodumi
Yorodumi- PDB-1c7m: SOLUTION STRUCTURE OF THE FUNCTIONAL DOMAIN OF PARACOCCUS DENITRI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1c7m | ||||||
|---|---|---|---|---|---|---|---|
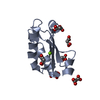
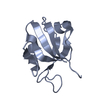
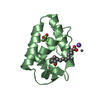
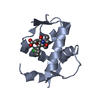
| Title | SOLUTION STRUCTURE OF THE FUNCTIONAL DOMAIN OF PARACOCCUS DENITRIFICANS CYTOCHROME C552 IN THE REDUCED STATE | ||||||
 Components Components | PROTEIN (CYTOCHROME C552) | ||||||
 Keywords Keywords |  ELECTRON TRANSPORT / CYTOCHROME C552 / ELECTRON TRANSPORT / CYTOCHROME C552 /  HEME / REDOX STATES / ISOTOPE ENRICHMENT {15N} / HEME / REDOX STATES / ISOTOPE ENRICHMENT {15N} /  NMR SPECTROSCOPY / SOLUTION STRUCTURE NMR SPECTROSCOPY / SOLUTION STRUCTURE | ||||||
| Function / homology |  Function and homology information Function and homology information electron transfer activity / electron transfer activity /  heme binding / heme binding /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Paracoccus denitrificans (bacteria) Paracoccus denitrificans (bacteria) | ||||||
| Method |  SOLUTION NMR / DISTANCE GEOMETRY, ENERGY MINIMIZATION SOLUTION NMR / DISTANCE GEOMETRY, ENERGY MINIMIZATION | ||||||
 Authors Authors | Pristovsek, P. / Luecke, C. / Reincke, B. / Ludwig, B. / Rueterjans, H. | ||||||
 Citation Citation |  Journal: Eur.J.Biochem. / Year: 2000 Journal: Eur.J.Biochem. / Year: 2000Title: Solution structure of the functional domain of Paracoccus denitrificans cytochrome c552 in the reduced state. Authors: Pristovsek, P. / Lucke, C. / Reincke, B. / Ludwig, B. / Ruterjans, H. #1:  Journal: J.Mol.Biol. / Year: 2000 Journal: J.Mol.Biol. / Year: 2000Title: Structure of the Soluble Domain of Cytochrome C552 from Paracoccus Denitrificans in the Oxidized and Reduced States Authors: Harrenga, A. / Reincke, B. / Rueterjans, H. / Ludwig, B. / Michel, H. #2:  Journal: Biochim.Biophys.Acta / Year: 1999 Journal: Biochim.Biophys.Acta / Year: 1999Title: Heterologous Expression of Soluble Fragments of Cytochrome C552 Acting as Electron Donor to the Paracoccus Denitrificans Cytochrome C Oxidase Authors: Reincke, B. / Thoeny-Meyer, L. / Dannehl, C. / Odenwald, A. / Aidim, M. / Witt, H. / Rueterjans, H. / Ludwig, B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1c7m.cif.gz 1c7m.cif.gz | 605 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1c7m.ent.gz pdb1c7m.ent.gz | 502.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1c7m.json.gz 1c7m.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c7/1c7m https://data.pdbj.org/pub/pdb/validation_reports/c7/1c7m ftp://data.pdbj.org/pub/pdb/validation_reports/c7/1c7m ftp://data.pdbj.org/pub/pdb/validation_reports/c7/1c7m | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 10553.977 Da / Num. of mol.: 1 / Fragment: SOLUBLE FUNCTIONAL DOMAIN Source method: isolated from a genetically manipulated source Details: COVALENT THIOETHER LINKAGES FROM HEME COFACTOR TO BOTH CYS14 AND CYS17 Source: (gene. exp.)   Paracoccus denitrificans (bacteria) / Strain: PD1235 / Description: HETEROLOGOUS EXPRESSION / Cellular location: PERIPLASM Paracoccus denitrificans (bacteria) / Strain: PD1235 / Description: HETEROLOGOUS EXPRESSION / Cellular location: PERIPLASM / Gene: CYCM / Plasmid: PET22B+ / Species (production host): Escherichia coli / Cellular location (production host): PERIPLASM / Production host: / Gene: CYCM / Plasmid: PET22B+ / Species (production host): Escherichia coli / Cellular location (production host): PERIPLASM / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21 (DE3) / References: UniProt: P54820 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21 (DE3) / References: UniProt: P54820 |
|---|---|
| #2: Chemical | ChemComp-HEC /  Heme C Heme C |
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||||||
| NMR details | Text: THE EXPERIMENTS WERE PERFORMED WITH REDUCED UNLABELED OR 15N-LABELED CYTOCHROME C552. |
- Sample preparation
Sample preparation
| Details | Contents: 20 MM PHOSPHATE, 90% H2O/ 10% D2O |
|---|---|
| Sample conditions | pH: 5.50 / Temperature: 298.00 K |
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: DISTANCE GEOMETRY, ENERGY MINIMIZATION / Software ordinal: 1 / Details: ENERGY MINIMIZATION. | ||||||||||||||||
| NMR ensemble | Conformer selection criteria: LEAST RESTRAINT VIOLATIONS / Conformers calculated total number: 300 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller







 PDBj
PDBj









