[English] 日本語
 Yorodumi
Yorodumi- EMDB-41784: PRD-0038 RBD bound to Rhinolophus alcyone ACE2 (local refinement) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | PRD-0038 RBD bound to Rhinolophus alcyone ACE2 (local refinement) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Sarbecoviruses /  Spike glycoprotein / Spike glycoprotein /  fusion protein / fusion protein /  neutralizing antibodies / neutralizing antibodies /  Structural Genomics / Seattle Structural Genomics Center for Infectious Disease / SSGCID / Structural Genomics / Seattle Structural Genomics Center for Infectious Disease / SSGCID /  inhibitor / inhibitor /  VIRAL PROTEIN VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information Hydrolases; Acting on peptide bonds (peptidases) / peptidyl-dipeptidase activity / Hydrolases; Acting on peptide bonds (peptidases) / peptidyl-dipeptidase activity /  carboxypeptidase activity / carboxypeptidase activity /  cilium / cilium /  metallopeptidase activity / metallopeptidase activity /  proteolysis / extracellular region / proteolysis / extracellular region /  metal ion binding / metal ion binding /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Sarbecovirus / Sarbecovirus /  Rhinolophus alcyone (Halcyon horseshoe bat) Rhinolophus alcyone (Halcyon horseshoe bat) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Park YJ / Veesler D / Seattle Structural Genomics Center for Infectious Disease (SSGCID) | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2023 Journal: Cell Host Microbe / Year: 2023Title: Broad receptor tropism and immunogenicity of a clade 3 sarbecovirus. Authors: Jimin Lee / Samantha K Zepeda / Young-Jun Park / Ashley L Taylor / Joel Quispe / Cameron Stewart / Elizabeth M Leaf / Catherine Treichel / Davide Corti / Neil P King / Tyler N Starr / David Veesler /   Abstract: Although Rhinolophus bats harbor diverse clade 3 sarbecoviruses, the structural determinants of receptor tropism along with the antigenicity of their spike (S) glycoproteins remain uncharacterized. ...Although Rhinolophus bats harbor diverse clade 3 sarbecoviruses, the structural determinants of receptor tropism along with the antigenicity of their spike (S) glycoproteins remain uncharacterized. Here, we show that the African Rhinolophus bat clade 3 sarbecovirus PRD-0038 S has a broad angiotensin-converting enzyme 2 (ACE2) usage and that receptor-binding domain (RBD) mutations further expand receptor promiscuity and enable human ACE2 utilization. We determine a cryo-EM structure of the PRD-0038 RBD bound to Rhinolophus alcyone ACE2, explaining receptor tropism and highlighting differences with SARS-CoV-1 and SARS-CoV-2. Characterization of PRD-0038 S using cryo-EM and monoclonal antibody reactivity reveals its distinct antigenicity relative to SARS-CoV-2 and identifies PRD-0038 cross-neutralizing antibodies for pandemic preparedness. PRD-0038 S vaccination elicits greater titers of antibodies cross-reacting with vaccine-mismatched clade 2 and clade 1a sarbecoviruses compared with SARS-CoV-2 S due to broader antigenic targeting, motivating the inclusion of clade 3 antigens in next-generation vaccines for enhanced resilience to viral evolution. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41784.map.gz emd_41784.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41784-v30.xml emd-41784-v30.xml emd-41784.xml emd-41784.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41784.png emd_41784.png | 93.9 KB | ||
| Filedesc metadata |  emd-41784.cif.gz emd-41784.cif.gz | 6.3 KB | ||
| Others |  emd_41784_additional_1.map.gz emd_41784_additional_1.map.gz emd_41784_half_map_1.map.gz emd_41784_half_map_1.map.gz emd_41784_half_map_2.map.gz emd_41784_half_map_2.map.gz | 62.7 MB 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41784 http://ftp.pdbj.org/pub/emdb/structures/EMD-41784 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41784 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41784 | HTTPS FTP |
-Related structure data
| Related structure data |  8u0tMC  8u29C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41784.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41784.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_41784_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
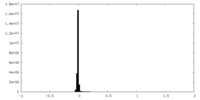
| Density Histograms |
-Half map: #2
| File | emd_41784_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
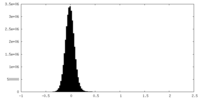
| Density Histograms |
-Half map: #1
| File | emd_41784_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
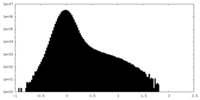
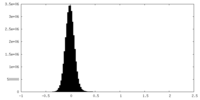
| Density Histograms |
- Sample components
Sample components
-Entire : PRD-0038 RBD bound to R. alcyone ACE2
| Entire | Name: PRD-0038 RBD bound to R. alcyone ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: PRD-0038 RBD bound to R. alcyone ACE2
| Supramolecule | Name: PRD-0038 RBD bound to R. alcyone ACE2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Sarbecovirus Sarbecovirus |
-Macromolecule #1: Angiotensin-converting enzyme
| Macromolecule | Name: Angiotensin-converting enzyme / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhinolophus alcyone (Halcyon horseshoe bat) Rhinolophus alcyone (Halcyon horseshoe bat) |
| Molecular weight | Theoretical: 89.075398 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSGSSWLFLS LVAVAAAQST PEDLAKIFLD NFNSEAENLS HQSSLASWEY NTNISDENIQ KMDEAGAKWS DFYETQSKHA KNFSLEEIH NDTVKLQLQI LQQSGSPVLS EDKSKRLNSI LNAMSTIYST GKVCRPNNPQ ECLLLEPGLD NIMGTSKDYN E RLWAWEGW ...String: MSGSSWLFLS LVAVAAAQST PEDLAKIFLD NFNSEAENLS HQSSLASWEY NTNISDENIQ KMDEAGAKWS DFYETQSKHA KNFSLEEIH NDTVKLQLQI LQQSGSPVLS EDKSKRLNSI LNAMSTIYST GKVCRPNNPQ ECLLLEPGLD NIMGTSKDYN E RLWAWEGW RAEVGKQLRP LYEEYVVLKN EMARGYHYED YGDYWRRDYE TEGSPDLEYS RDQLTKDVER IFAEIKPLYE QL HAYVRTK LMDTYPFHIS PTGCLPAHLL GDMWGRFWTN LYPLTVPFAQ KPNIDVTDAM LNQTWDAKRI FKEAEKFFVS IGL PHMTEG FWNNSMLTDP GDGRKVVCHP TAWDLGKGDF RIKMCTKVTM EDFLTAHHEM GHIQYDMAYA SQPYLLRNGA NEGF HEAVG EVMSLSVATP KHLKTMGLLS PDFLEDNETE INFLFKQALT IVGTLPFTYM LEKWRWMVFK GEIPKEEWMT KWWEM KRKI VGVVEPVPHD ETYCDPASLF HVANDYSFIR YYTRTIFEFQ FHEALCRIAK HDGPLHKCDI SNSTDAGKKL HQMLSV GKS QPWTSVLKDF VDSKDMDVGP LLRYFEPLYT WLKEQNRNSF VGWNTDWSPY ADQSIKVRIS LKSALGEKAY EWNNNEM YL FRSSVAYAMR EYFLKTKNQT ILFGEEDVWV SNLKPRISFN FYVTSPRNLS DIIPRPEVEG AIRMSRSRIN DAFRLDDN S LEFLGIQPTL GPPYQPPVTH HHHHHHHGGS SGLNDIFEAQ KIEWHE UniProtKB:  Angiotensin-converting enzyme Angiotensin-converting enzyme |
-Macromolecule #2: PRD-0038
| Macromolecule | Name: PRD-0038 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Sarbecovirus Sarbecovirus |
| Molecular weight | Theoretical: 28.584332 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGILPSPGMP ALLSLVSLLS VLLMGCVARF PNITNLCPFG QVFNASKFPS VYAWERLRIS DCVADYSVLY NSSSSFSTFK CYGVSPTKL NDLCFSSVYA DYFVVKGDDV RQIAPAQTGV IADYNYKLPD DFTGCVLAWN TNSVDSKQGN NFYYRLFRHG K IKPYERDI ...String: MGILPSPGMP ALLSLVSLLS VLLMGCVARF PNITNLCPFG QVFNASKFPS VYAWERLRIS DCVADYSVLY NSSSSFSTFK CYGVSPTKL NDLCFSSVYA DYFVVKGDDV RQIAPAQTGV IADYNYKLPD DFTGCVLAWN TNSVDSKQGN NFYYRLFRHG K IKPYERDI SNVLYNSAGG TCSSTSQLGC YEPLKSYGFT PTVGVGYQPY RVVVLSFELL NAPATVCGPK KSTHHHHHHH HG GSSGLND IFEAQKIEWH E |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 9 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 284934 |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X