+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of DDB1dB:CRBN:Pomalidomide:SD40 | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  ubiquitin / ubiquitin /  CRBN / CRBN /  directed evolution / directed evolution /  Zinc finger / IMiD / Zinc finger / IMiD /  Molecular Glue / Molecular Glue /  TRANSFERASE TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of monoatomic ion transmembrane transport / lymphocyte differentiation / positive regulation by virus of viral protein levels in host cell / epigenetic programming in the zygotic pronuclei / spindle assembly involved in female meiosis / Cul4-RING E3 ubiquitin ligase complex / UV-damage excision repair / biological process involved in interaction with symbiont / regulation of mitotic cell cycle phase transition / WD40-repeat domain binding ...negative regulation of monoatomic ion transmembrane transport / lymphocyte differentiation / positive regulation by virus of viral protein levels in host cell / epigenetic programming in the zygotic pronuclei / spindle assembly involved in female meiosis / Cul4-RING E3 ubiquitin ligase complex / UV-damage excision repair / biological process involved in interaction with symbiont / regulation of mitotic cell cycle phase transition / WD40-repeat domain binding / Cul4A-RING E3 ubiquitin ligase complex / NOTCH3 Intracellular Domain Regulates Transcription / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / detection of maltose stimulus /  maltose binding / maltose transport complex / maltose transport / maltodextrin transmembrane transport / negative regulation of reproductive process / negative regulation of developmental process / locomotory exploration behavior / cullin family protein binding / mesoderm development / carbohydrate transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / positive regulation of Wnt signaling pathway / carbohydrate transport / viral release from host cell / ectopic germ cell programmed cell death / negative regulation of protein-containing complex assembly / positive regulation of viral genome replication / pericentric heterochromatin / positive regulation of gluconeogenesis / ATP-binding cassette (ABC) transporter complex / cell chemotaxis / maltose binding / maltose transport complex / maltose transport / maltodextrin transmembrane transport / negative regulation of reproductive process / negative regulation of developmental process / locomotory exploration behavior / cullin family protein binding / mesoderm development / carbohydrate transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / positive regulation of Wnt signaling pathway / carbohydrate transport / viral release from host cell / ectopic germ cell programmed cell death / negative regulation of protein-containing complex assembly / positive regulation of viral genome replication / pericentric heterochromatin / positive regulation of gluconeogenesis / ATP-binding cassette (ABC) transporter complex / cell chemotaxis /  erythrocyte differentiation / proteasomal protein catabolic process / Recognition of DNA damage by PCNA-containing replication complex / nucleotide-excision repair / positive regulation of protein-containing complex assembly / DNA Damage Recognition in GG-NER / erythrocyte differentiation / proteasomal protein catabolic process / Recognition of DNA damage by PCNA-containing replication complex / nucleotide-excision repair / positive regulation of protein-containing complex assembly / DNA Damage Recognition in GG-NER /  regulation of circadian rhythm / Dual Incision in GG-NER / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / regulation of circadian rhythm / Dual Incision in GG-NER / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex /  Wnt signaling pathway / Formation of Incision Complex in GG-NER / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / positive regulation of protein catabolic process / cellular response to UV / rhythmic process / protein-macromolecule adaptor activity / site of double-strand break / Wnt signaling pathway / Formation of Incision Complex in GG-NER / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / positive regulation of protein catabolic process / cellular response to UV / rhythmic process / protein-macromolecule adaptor activity / site of double-strand break /  Neddylation / outer membrane-bounded periplasmic space / chromatin organization / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / Potential therapeutics for SARS / transmembrane transporter binding / Neddylation / outer membrane-bounded periplasmic space / chromatin organization / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / Potential therapeutics for SARS / transmembrane transporter binding /  chromosome, telomeric region / damaged DNA binding / chromosome, telomeric region / damaged DNA binding /  periplasmic space / protein ubiquitination / periplasmic space / protein ubiquitination /  cell cycle / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / protein domain specific binding / cell cycle / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / protein domain specific binding /  DNA repair / negative regulation of DNA-templated transcription / apoptotic process / DNA damage response / protein-containing complex binding / DNA repair / negative regulation of DNA-templated transcription / apoptotic process / DNA damage response / protein-containing complex binding /  nucleolus / regulation of transcription by RNA polymerase II / negative regulation of apoptotic process / perinuclear region of cytoplasm / protein-containing complex / nucleolus / regulation of transcription by RNA polymerase II / negative regulation of apoptotic process / perinuclear region of cytoplasm / protein-containing complex /  DNA binding / DNA binding /  extracellular space / extracellular exosome / extracellular space / extracellular exosome /  nucleoplasm / nucleoplasm /  membrane / identical protein binding / membrane / identical protein binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
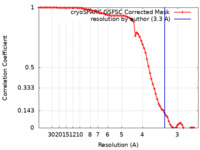
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Roy Burman SS / Hunkeler M / Fischer ES | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2024 Journal: Science / Year: 2024Title: Continuous evolution of compact protein degradation tags regulated by selective molecular glues. Authors: Jaron A M Mercer / Stephan J DeCarlo / Shourya S Roy Burman / Vedagopuram Sreekanth / Andrew T Nelson / Moritz Hunkeler / Peter J Chen / Katherine A Donovan / Praveen Kokkonda / Praveen K ...Authors: Jaron A M Mercer / Stephan J DeCarlo / Shourya S Roy Burman / Vedagopuram Sreekanth / Andrew T Nelson / Moritz Hunkeler / Peter J Chen / Katherine A Donovan / Praveen Kokkonda / Praveen K Tiwari / Veronika M Shoba / Arghya Deb / Amit Choudhary / Eric S Fischer / David R Liu /  Abstract: Conditional protein degradation tags (degrons) are usually >100 amino acids long or are triggered by small molecules with substantial off-target effects, thwarting their use as specific modulators of ...Conditional protein degradation tags (degrons) are usually >100 amino acids long or are triggered by small molecules with substantial off-target effects, thwarting their use as specific modulators of endogenous protein levels. We developed a phage-assisted continuous evolution platform for molecular glue complexes (MG-PACE) and evolved a 36-amino acid zinc finger (ZF) degron (SD40) that binds the ubiquitin ligase substrate receptor cereblon in complex with PT-179, an orthogonal thalidomide derivative. Endogenous proteins tagged in-frame with SD40 using prime editing are degraded by otherwise inert PT-179. Cryo-electron microscopy structures of SD40 in complex with ligand-bound cereblon revealed mechanistic insights into the molecular basis of SD40's activity and specificity. Our efforts establish a system for continuous evolution of molecular glue complexes and provide ZF tags that overcome shortcomings associated with existing degrons. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41423.map.gz emd_41423.map.gz | 40.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41423-v30.xml emd-41423-v30.xml emd-41423.xml emd-41423.xml | 41.2 KB 41.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41423_fsc.xml emd_41423_fsc.xml | 7.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_41423.png emd_41423.png | 90.3 KB | ||
| Masks |  emd_41423_msk_1.map emd_41423_msk_1.map | 42.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-41423.cif.gz emd-41423.cif.gz | 8.7 KB | ||
| Others |  emd_41423_additional_1.map.gz emd_41423_additional_1.map.gz emd_41423_additional_2.map.gz emd_41423_additional_2.map.gz emd_41423_additional_3.map.gz emd_41423_additional_3.map.gz emd_41423_additional_4.map.gz emd_41423_additional_4.map.gz emd_41423_additional_5.map.gz emd_41423_additional_5.map.gz emd_41423_additional_6.map.gz emd_41423_additional_6.map.gz emd_41423_additional_7.map.gz emd_41423_additional_7.map.gz emd_41423_half_map_1.map.gz emd_41423_half_map_1.map.gz emd_41423_half_map_2.map.gz emd_41423_half_map_2.map.gz | 1.1 MB 39.8 MB 39.8 MB 38.4 MB 21.8 MB 38.5 MB 40.5 MB 39.8 MB 39.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41423 http://ftp.pdbj.org/pub/emdb/structures/EMD-41423 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41423 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41423 | HTTPS FTP |
-Related structure data
| Related structure data |  8tnpMC  8tnqC  8tnrC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41423.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41423.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.277 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Mask #1
+Additional map: main map, local resolution-filtered
+Additional map: from local refinement with CRBN mask, half map A
+Additional map: from local refinement with CRBN mask, half map B
+Additional map: map from local refinement, post-processed with deepEMhancer
+Additional map: unsharpened main map
+Additional map: main map, post-processed by deepEMhancer
+Additional map: from local refinement with CRBN mask
+Half map: half map A
+Half map: half map B
- Sample components
Sample components
-Entire : Ternary Complex of DDB1dB:CRBN:pomalidomide with SD40
| Entire | Name: Ternary Complex of DDB1dB:CRBN:pomalidomide with SD40 |
|---|---|
| Components |
|
-Supramolecule #1: Ternary Complex of DDB1dB:CRBN:pomalidomide with SD40
| Supramolecule | Name: Ternary Complex of DDB1dB:CRBN:pomalidomide with SD40 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Molecular weight | Theoretical: 201 KDa |
-Macromolecule #1: Protein cereblon
| Macromolecule | Name: Protein cereblon / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.144594 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MDYKDDDDKS AVDENLYFQG GGRGGSAHIV MVDAYKPTKG GSGMAGEGDQ QDAAHNMGNH LPLLPAESEE EDEMEVEDQD SKEAKKPNI INFDTSLPTS HTYLGADMEE FHGRTLHDDD SCQVIPVLPQ VMMILIPGQT LPLQLFHPQE VSMVRNLIQK D RTFAVLAY ...String: MDYKDDDDKS AVDENLYFQG GGRGGSAHIV MVDAYKPTKG GSGMAGEGDQ QDAAHNMGNH LPLLPAESEE EDEMEVEDQD SKEAKKPNI INFDTSLPTS HTYLGADMEE FHGRTLHDDD SCQVIPVLPQ VMMILIPGQT LPLQLFHPQE VSMVRNLIQK D RTFAVLAY SNVQEREAQF GTTAEIYAYR EEQDFGIEIV KVKAIGRQRF KVLELRTQSD GIQQAKVQIL PECVLPSTMS AV QLESLNK CQIFPSKPVS REDQCSYKWW QKYQKRKFHC ANLTSWPRWL YSLYDAETLM DRIKKQLREW DENLKDDSLP SNP IDFSYR VAACLPIDDV LRIQLLKIGS AIQRLRCELD IMNKCTSLCC KQCQETEITT KNEIFSLSLC GPMAAYVNPH GYVH ETLTV YKACNLNLIG RPSTEHSWFP GYAWTVAQCK ICASHIGWKF TATKKDMSPQ KFWGLTRSAL LPTIPDTEDE ISPDK VILC L UniProtKB: Protein cereblon |
-Macromolecule #2: DNA damage-binding protein 1
| Macromolecule | Name: DNA damage-binding protein 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 96.033945 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGSSHHHHHH SAVDENLYFQ GGGRMSYNYV VTAQKPTAVN GCVTGHFTSA EDLNLLIAKN TRLEIYVVTA EGLRPVKEVG MYGKIAVME LFRPKGESKD LLFILTAKYN ACILEYKQSG ESIDIITRAH GNVQDRIGRP SETGIIGIID PECRMIGLRL Y DGLFKVIP ...String: MGSSHHHHHH SAVDENLYFQ GGGRMSYNYV VTAQKPTAVN GCVTGHFTSA EDLNLLIAKN TRLEIYVVTA EGLRPVKEVG MYGKIAVME LFRPKGESKD LLFILTAKYN ACILEYKQSG ESIDIITRAH GNVQDRIGRP SETGIIGIID PECRMIGLRL Y DGLFKVIP LDRDNKELKA FNIRLEELHV IDVKFLYGCQ APTICFVYQD PQGRHVKTYE VSLREKEFNK GPWKQENVEA EA SMVIAVP EPFGGAIIIG QESITYHNGD KYLAIAPPII KQSTIVCHNR VDPNGSRYLL GDMEGRLFML LLEKEEQMDG TVT LKDLRV ELLGETSIAE CLTYLDNGVV FVGSRLGDSQ LVKLNVDSNE QGSYVVAMET FTNLGPIVDM CVVDLERQGQ GQLV TCSGA FKEGSLRIIR NGIGGNGNSG EIQKLHIRTV PLYESPRKIC YQEVSQCFGV LSSRIEVQDT SGGTTALRPS ASTQA LSSS VSSSKLFSSS TAPHETSFGE EVEVHNLLII DQHTFEVLHA HQFLQNEYAL SLVSCKLGKD PNTYFIVGTA MVYPEE AEP KQGRIVVFQY SDGKLQTVAE KEVKGAVYSM VEFNGKLLAS INSTVRLYEW TTEKELRTEC NHYNNIMALY LKTKGDF IL VGDLMRSVLL LAYKPMEGNF EEIARDFNPN WMSAVEILDD DNFLGAENAF NLFVCQKDSA ATTDEERQHL QEVGLFHL G EFVNVFCHGS LVMQNLGETS TPTQGSVLFG TVNGMIGLVT SLSESWYNLL LDMQNRLNKV IKSVGKIEHS FWRSFHTER KTEPATGFID GDLIESFLDI SRPKMQEVVA NLQYDDGSGM KREATADDLI KVVEELTRIH UniProtKB: DNA damage-binding protein 1, DNA damage-binding protein 1 |
-Macromolecule #3: Maltose/maltodextrin-binding periplasmic protein,SD40
| Macromolecule | Name: Maltose/maltodextrin-binding periplasmic protein,SD40 / type: protein_or_peptide / ID: 3 Details: IZKF1/ZFP91 fusion construct that was further engineered to enhance binding of cereblon/DDB1 in the presence of IMiD derivatives Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.466012 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MGLNDIFEAQ KIEWHEGSSH HHHHHGSSKI EEGKLVIWIN GDKGYNGLAE VGKKFEKDTG IKVTVEHPDK LEEKFPQVAA TGDGPDIIF WAHDRFGGYA QSGLLAEITP DKAFQDKLYP FTWDAVRYNG KLIAYPIAVE ALSLIYNKDL LPNPPKTWEE I PALDKELK ...String: MGLNDIFEAQ KIEWHEGSSH HHHHHGSSKI EEGKLVIWIN GDKGYNGLAE VGKKFEKDTG IKVTVEHPDK LEEKFPQVAA TGDGPDIIF WAHDRFGGYA QSGLLAEITP DKAFQDKLYP FTWDAVRYNG KLIAYPIAVE ALSLIYNKDL LPNPPKTWEE I PALDKELK AKGKSALMFN LQEPYFTWPL IAADGGYAFK YENGKYDIKD VGVDNAGAKA GLTFLVDLIK NKHMNADTDY SI AEAAFNK GETAMTINGP WAWSNIDTSK VNYGVTVLPT FKGQPSKPFV GVLSAGINAA SPNKELAKEF LENYLLTDEG LEA VNKDKP LGAVALKSYE EELAKDPRIA ATMENAQKGE IMPNIPQMSA FWYAVRTAVI NAASGRQTVD EALKDAQTRI TKLE VLFQG PDYKDDDDKS GGGGLLLFCP ICGFTCRQKG NLLRHINLHT GEKLFKYHLY UniProtKB: Maltose/maltodextrin-binding periplasmic protein, DNA-binding protein Ikaros |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #5: S-Pomalidomide
| Macromolecule | Name: S-Pomalidomide / type: ligand / ID: 5 / Number of copies: 1 / Formula: Y70 |
|---|---|
| Molecular weight | Theoretical: 273.244 Da |
| Chemical component information |  ChemComp-Y70: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2625 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
Details: 20 mM HEPES/NaOH pH 7.0, 150 mM NaCl, and 3 mM TCEP. DMSO concentrations were kept below 2% (v/v) | ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY / Support film - Film thickness: 50 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa Details: Grids (Quantifoil UltrAuFoil R 1.2/1.3) were glow discharged in PELCO easiGlow (20 mA, 120s, 39 Pa) | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 283 K / Instrument: LEICA EM GP Details: Leica EM-GP plunge freezer with chamber conditions of 10 C and 90% relative humidity. Grids were first pre-incubated with 4 uL of 10 uM CRBN-agnostic IKZF1_140-196_Q146A,G151N for 1 minute ...Details: Leica EM-GP plunge freezer with chamber conditions of 10 C and 90% relative humidity. Grids were first pre-incubated with 4 uL of 10 uM CRBN-agnostic IKZF1_140-196_Q146A,G151N for 1 minute and then blotted from behind for 4 s. Immediately, 4 uL of mixture 1 diluted 10-fold--with the dilution buffer during the 1-minute incubation time--was applied to the grids before blotting for 4 s and plunging into liquid ethane at -181 C.. | ||||||||||||
| Details | DDB1dB_CRBN, pomalidomide, and SD40 were mixed and incubated on ice for 1 hour at final concentration of 10.5, 105, and 21 uM, respectively. Then diluted 10-fold before blotting. |
- Electron microscopy
Electron microscopy
| Microscope | TFS TALOS |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 36000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 2524 / Average exposure time: 4.993 sec. / Average electron dose: 53.8 e/Å2 Details: Movies (50 frames) collected using beam-shift with 9 holes per stage position (3x3 pattern) |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Details | Phenix real-space refinement without rigid body | ||||||||
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 102 / Target criteria: CC | ||||||||
| Output model |  PDB-8tnp: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)