+ Open data
Open data
- Basic information
Basic information
| Entry | 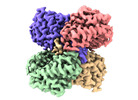 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
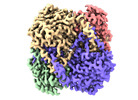
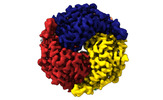
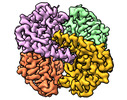
| Title | human liver mitochondrial Aldehyde dehydrogenase ALDH2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  human / human /  liver / liver /  mitochondrial / mitochondrial /  Aldehyde dehydrogenase / ALDH2 / Aldehyde dehydrogenase / ALDH2 /  OXIDOREDUCTASE OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationMetabolism of serotonin / nitroglycerin reductase activity / regulation of dopamine biosynthetic process / regulation of serotonin biosynthetic process /  phenylacetaldehyde dehydrogenase activity / aldehyde catabolic process / alcohol metabolic process / aldehyde dehydrogenase [NAD(P)+] activity / ethanol catabolic process / Ethanol oxidation ...Metabolism of serotonin / nitroglycerin reductase activity / regulation of dopamine biosynthetic process / regulation of serotonin biosynthetic process / phenylacetaldehyde dehydrogenase activity / aldehyde catabolic process / alcohol metabolic process / aldehyde dehydrogenase [NAD(P)+] activity / ethanol catabolic process / Ethanol oxidation ...Metabolism of serotonin / nitroglycerin reductase activity / regulation of dopamine biosynthetic process / regulation of serotonin biosynthetic process /  phenylacetaldehyde dehydrogenase activity / aldehyde catabolic process / alcohol metabolic process / aldehyde dehydrogenase [NAD(P)+] activity / ethanol catabolic process / Ethanol oxidation / phenylacetaldehyde dehydrogenase activity / aldehyde catabolic process / alcohol metabolic process / aldehyde dehydrogenase [NAD(P)+] activity / ethanol catabolic process / Ethanol oxidation /  carboxylesterase activity / glyceraldehyde-3-phosphate dehydrogenase (NAD+) (non-phosphorylating) activity / carboxylesterase activity / glyceraldehyde-3-phosphate dehydrogenase (NAD+) (non-phosphorylating) activity /  aldehyde dehydrogenase (NAD+) / aldehyde dehydrogenase (NAD+) /  aldehyde dehydrogenase (NAD+) activity / Smooth Muscle Contraction / NAD binding / aldehyde dehydrogenase (NAD+) activity / Smooth Muscle Contraction / NAD binding /  electron transfer activity / carbohydrate metabolic process / electron transfer activity / carbohydrate metabolic process /  mitochondrial matrix / mitochondrial matrix /  mitochondrion / extracellular exosome mitochondrion / extracellular exosomeSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.66 Å cryo EM / Resolution: 2.66 Å | |||||||||
 Authors Authors | Zhang Z | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell Proteomics / Year: 2023 Journal: Mol Cell Proteomics / Year: 2023Title: High-Resolution Structural Proteomics of Mitochondria Using the 'Build and Retrieve' Methodology. Authors: Zhemin Zhang / Marios L Tringides / Christopher E Morgan / Masaru Miyagi / Jason A Mears / Charles L Hoppel / Edward W Yu /  Abstract: The application of integrated systems biology to the field of structural biology is a promising new direction, although it is still in the infant stages of development. Here we report the use of ...The application of integrated systems biology to the field of structural biology is a promising new direction, although it is still in the infant stages of development. Here we report the use of single particle cryo-EM to identify multiple proteins from three enriched heterogeneous fractions prepared from human liver mitochondrial lysate. We simultaneously identify and solve high-resolution structures of nine essential mitochondrial enzymes with key metabolic functions, including fatty acid catabolism, reactive oxidative species clearance, and amino acid metabolism. Our methodology also identified multiple distinct members of the acyl-CoA dehydrogenase family. This work highlights the potential of cryo-EM to explore tissue proteomics at the atomic level. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40493.map.gz emd_40493.map.gz | 83.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40493-v30.xml emd-40493-v30.xml emd-40493.xml emd-40493.xml | 13.2 KB 13.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40493_fsc.xml emd_40493_fsc.xml | 12.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_40493.png emd_40493.png | 105.1 KB | ||
| Filedesc metadata |  emd-40493.cif.gz emd-40493.cif.gz | 5.1 KB | ||
| Others |  emd_40493_half_map_1.map.gz emd_40493_half_map_1.map.gz emd_40493_half_map_2.map.gz emd_40493_half_map_2.map.gz | 154.2 MB 154.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40493 http://ftp.pdbj.org/pub/emdb/structures/EMD-40493 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40493 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40493 | HTTPS FTP |
-Related structure data
| Related structure data |  8shsMC  8sgpC  8sgrC  8sgsC  8sgvC  8sk6C  8sk8C  8skrC  8sksC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40493.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40493.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.8255 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_40493_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40493_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ALDH2
| Entire | Name: ALDH2 |
|---|---|
| Components |
|
-Supramolecule #1: ALDH2
| Supramolecule | Name: ALDH2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Aldehyde dehydrogenase, mitochondrial
| Macromolecule | Name: Aldehyde dehydrogenase, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number:  aldehyde dehydrogenase (NAD+) aldehyde dehydrogenase (NAD+) |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.441047 KDa |
| Sequence | String: MLRAAARFGP RLGRRLLSAA ATQAVPAPNQ QPEVFCNQIF INNEWHDAVS RKTFPTVNPS TGEVICQVAE GDKEDVDKAV KAARAAFQL GSPWRRMDAS HRGRLLNRLA DLIERDRTYL AALETLDNGK PYVISYLVDL DMVLKCLRYY AGWADKYHGK T IPIDGDFF ...String: MLRAAARFGP RLGRRLLSAA ATQAVPAPNQ QPEVFCNQIF INNEWHDAVS RKTFPTVNPS TGEVICQVAE GDKEDVDKAV KAARAAFQL GSPWRRMDAS HRGRLLNRLA DLIERDRTYL AALETLDNGK PYVISYLVDL DMVLKCLRYY AGWADKYHGK T IPIDGDFF SYTRHEPVGV CGQIIPWNFP LLMQAWKLGP ALATGNVVVM KVAEQTPLTA LYVANLIKEA GFPPGVVNIV PG FGPTAGA AIASHEDVDK VAFTGSTEIG RVIQVAAGSS NLKRVTLELG GKSPNIIMSD ADMDWAVEQA HFALFFNQGQ CCC AGSRTF VQEDIYDEFV ERSVARAKSR VVGNPFDSKT EQGPQVDETQ FKKILGYINT GKQEGAKLLC GGGIAADRGY FIQP TVFGD VQDGMTIAKE EIFGPVMQIL KFKTIEEVVG RANNSTYGLA AAVFTKDLDK ANYLSQALQA GTVWVNCYDV FGAQS PFGG YKMSGSGREL GEYGLQAYTE VKTVTVKVPQ KNS UniProtKB:  Aldehyde dehydrogenase, mitochondrial Aldehyde dehydrogenase, mitochondrial |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 Bright-field microscopy / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 35.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




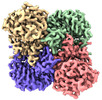
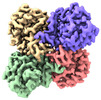
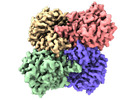








 Z
Z Y
Y X
X


















