+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | NuA4 Histone Acetyltransferase complex - Upper lobe | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NuA4 /  histone acetyltransferase complex / histone acetyltransferase complex /  Epigenetics / Epigenetics /  DNA repair / DNA repair /  TRANSCRIPTION TRANSCRIPTION | |||||||||
| Biological species |  Komagataella phaffii GS115 (fungus) Komagataella phaffii GS115 (fungus) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.34 Å cryo EM / Resolution: 3.34 Å | |||||||||
 Authors Authors | Frechard A / Hexnerova R / Crucifix C / Papai G / Smirnova E / Faux C / Lo Ying Ping F / Helmlinger D / Schultz P / Ben-shem A | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: The structure of the NuA4-Tip60 complex reveals the mechanism and importance of long-range chromatin modification. Authors: Alexander Fréchard / Céline Faux / Rozalie Hexnerova / Corinne Crucifix / Gabor Papai / Ekaterina Smirnova / Conor McKeon / Florie Lo Ying Ping / Dominique Helmlinger / Patrick Schultz / Adam Ben-Shem /  Abstract: Histone acetylation regulates most DNA transactions and is dynamically controlled by highly conserved enzymes. The only essential histone acetyltransferase (HAT) in yeast, Esa1, is part of the 1-MDa ...Histone acetylation regulates most DNA transactions and is dynamically controlled by highly conserved enzymes. The only essential histone acetyltransferase (HAT) in yeast, Esa1, is part of the 1-MDa NuA4 complex, which plays pivotal roles in both transcription and DNA-damage repair. NuA4 has the unique capacity to acetylate histone targets located several nucleosomes away from its recruitment site. Neither the molecular mechanism of this activity nor its physiological importance are known. Here we report the structure of the Pichia pastoris NuA4 complex, with its core resolved at 3.4-Å resolution. Three subunits, Epl1, Eaf1 and Swc4, intertwine to form a stable platform that coordinates all other modules. The HAT module is firmly anchored into the core while retaining the ability to stretch out over a long distance. We provide structural, biochemical and genetic evidence that an unfolded linker region of the Epl1 subunit is critical for this long-range activity. Specifically, shortening the Epl1 linker causes severe growth defects and reduced H4 acetylation levels over broad chromatin regions in fission yeast. Our work lays the foundations for a mechanistic understanding of NuA4's regulatory role and elucidates how its essential long-range activity is attained. #1:  Journal: Res Sq / Year: 2022 Journal: Res Sq / Year: 2022Title: The structure of the NuA4/Tip60 complex reveals the mechanism and importance of long-range chromatin modification Authors: Schultz P / Frechard A / Hexnerova R / Crucifix C / Papai G / Smirnova E / Faux C / Ping F / Helmlinger D / Ben-Shem A | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15066.map.gz emd_15066.map.gz | 320.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15066-v30.xml emd-15066-v30.xml emd-15066.xml emd-15066.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_15066.png emd_15066.png | 37.5 KB | ||
| Masks |  emd_15066_msk_1.map emd_15066_msk_1.map | 343 MB |  Mask map Mask map | |
| Others |  emd_15066_half_map_1.map.gz emd_15066_half_map_1.map.gz emd_15066_half_map_2.map.gz emd_15066_half_map_2.map.gz | 318.4 MB 318.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15066 http://ftp.pdbj.org/pub/emdb/structures/EMD-15066 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15066 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15066 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15066.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15066.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.862 Å | ||||||||||||||||||||||||||||||||||||
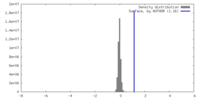
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15066_msk_1.map emd_15066_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
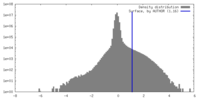
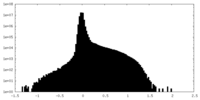
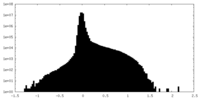
| Density Histograms |
-Half map: Half map B
| File | emd_15066_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_15066_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : NuA4 histone acetyltransferase complex
| Entire | Name: NuA4 histone acetyltransferase complex |
|---|---|
| Components |
|
-Supramolecule #1: NuA4 histone acetyltransferase complex
| Supramolecule | Name: NuA4 histone acetyltransferase complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  Komagataella phaffii GS115 (fungus) Komagataella phaffii GS115 (fungus) |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.25 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 279 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.8000000000000003 µm / Nominal defocus min: 1.4000000000000001 µm Bright-field microscopy / Nominal defocus max: 3.8000000000000003 µm / Nominal defocus min: 1.4000000000000001 µm |
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 52.8 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 1265830 |
|---|---|
| Startup model | Type of model: OTHER / Details: cryoSPARC ab-initio reconstruction |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.34 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 518386 |
 Movie
Movie Controller
Controller



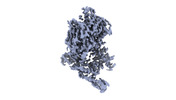









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)