+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7pc0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GABA-A receptor bound by a-Cobratoxin | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  neurotransmission / neurotransmission /  toxin / GABA toxin / GABA | |||||||||
| Function / homology |  Function and homology information Function and homology information GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / GABA-gated chloride ion channel activity / cellular response to histamine / GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / GABA-gated chloride ion channel activity / cellular response to histamine /  inhibitory synapse assembly / inhibitory synapse assembly /  GABA-A receptor activity / GABA-A receptor activity /  GABA-A receptor complex / acetylcholine receptor inhibitor activity / GABA-A receptor complex / acetylcholine receptor inhibitor activity /  neurotransmitter receptor activity ... neurotransmitter receptor activity ... GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / GABA-gated chloride ion channel activity / cellular response to histamine / GABA receptor complex / inhibitory extracellular ligand-gated monoatomic ion channel activity / GABA receptor activation / GABA-gated chloride ion channel activity / cellular response to histamine /  inhibitory synapse assembly / inhibitory synapse assembly /  GABA-A receptor activity / GABA-A receptor activity /  GABA-A receptor complex / acetylcholine receptor inhibitor activity / GABA-A receptor complex / acetylcholine receptor inhibitor activity /  neurotransmitter receptor activity / ion channel regulator activity / neurotransmitter receptor activity / ion channel regulator activity /  synaptic transmission, GABAergic / gamma-aminobutyric acid signaling pathway / roof of mouth development / Signaling by ERBB4 / synaptic transmission, GABAergic / gamma-aminobutyric acid signaling pathway / roof of mouth development / Signaling by ERBB4 /  chloride channel complex / GABA-ergic synapse / regulation of postsynaptic membrane potential / chloride transmembrane transport / dendrite membrane / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / cytoplasmic vesicle membrane / chloride channel complex / GABA-ergic synapse / regulation of postsynaptic membrane potential / chloride transmembrane transport / dendrite membrane / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / cytoplasmic vesicle membrane /  toxin activity / postsynapse / toxin activity / postsynapse /  postsynaptic membrane / neuron projection / postsynaptic membrane / neuron projection /  synapse / synapse /  signal transduction / extracellular region / identical protein binding / signal transduction / extracellular region / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Lama glama (llama) Lama glama (llama)  Homo sapiens (human) Homo sapiens (human)  Naja kaouthia (monocled cobra) Naja kaouthia (monocled cobra) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3 Å cryo EM / Resolution: 3 Å | |||||||||
 Authors Authors | Kasaragod, V.B. / Miller, P.S. | |||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Mechanisms of inhibition and activation of extrasynaptic αβ GABA receptors. Authors: Vikram Babu Kasaragod / Martin Mortensen / Steven W Hardwick / Ayla A Wahid / Valentina Dorovykh / Dimitri Y Chirgadze / Trevor G Smart / Paul S Miller /  Abstract: Type A GABA (γ-aminobutyric acid) receptors represent a diverse population in the mammalian brain, forming pentamers from combinations of α-, β-, γ-, δ-, ε-, ρ-, θ- and π-subunits. αβ, ...Type A GABA (γ-aminobutyric acid) receptors represent a diverse population in the mammalian brain, forming pentamers from combinations of α-, β-, γ-, δ-, ε-, ρ-, θ- and π-subunits. αβ, α4βδ, α6βδ and α5βγ receptors favour extrasynaptic localization, and mediate an essential persistent (tonic) inhibitory conductance in many regions of the mammalian brain. Mutations of these receptors in humans are linked to epilepsy and insomnia. Altered extrasynaptic receptor function is implicated in insomnia, stroke and Angelman and Fragile X syndromes, and drugs targeting these receptors are used to treat postpartum depression. Tonic GABAergic responses are moderated to avoid excessive suppression of neuronal communication, and can exhibit high sensitivity to Zn blockade, in contrast to synapse-preferring α1βγ, α2βγ and α3βγ receptor responses. Here, to resolve these distinctive features, we determined structures of the predominantly extrasynaptic αβ GABA receptor class. An inhibited state bound by both the lethal paralysing agent α-cobratoxin and Zn was used in comparisons with GABA-Zn and GABA-bound structures. Zn nullifies the GABA response by non-competitively plugging the extracellular end of the pore to block chloride conductance. In the absence of Zn, the GABA signalling response initially follows the canonical route until it reaches the pore. In contrast to synaptic GABA receptors, expansion of the midway pore activation gate is limited and it remains closed, reflecting the intrinsic low efficacy that characterizes the extrasynaptic receptor. Overall, this study explains distinct traits adopted by αβ receptors that adapt them to a role in tonic signalling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7pc0.cif.gz 7pc0.cif.gz | 378 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7pc0.ent.gz pdb7pc0.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7pc0.json.gz 7pc0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pc/7pc0 https://data.pdbj.org/pub/pdb/validation_reports/pc/7pc0 ftp://data.pdbj.org/pub/pdb/validation_reports/pc/7pc0 ftp://data.pdbj.org/pub/pdb/validation_reports/pc/7pc0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  13315MC  7pbdC  7pbzC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Gamma-aminobutyric acid receptor subunit alpha- ... , 2 types, 2 molecules AD
| #2: Protein | Mass: 41991.133 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GABRA1 / Production host: Homo sapiens (human) / Gene: GABRA1 / Production host:   Homo sapiens (human) / References: UniProt: P14867 Homo sapiens (human) / References: UniProt: P14867 |
|---|---|
| #4: Protein | Mass: 47059.648 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GABRA1 / Production host: Homo sapiens (human) / Gene: GABRA1 / Production host:   Homo sapiens (human) / References: UniProt: P14867 Homo sapiens (human) / References: UniProt: P14867 |
-Protein , 2 types, 5 molecules BCEKL
| #3: Protein | Mass: 51615.117 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GABRB3 / Production host: Homo sapiens (human) / Gene: GABRB3 / Production host:   Homo sapiens (human) / References: UniProt: P28472 Homo sapiens (human) / References: UniProt: P28472#5: Protein | Mass: 7842.094 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Naja kaouthia (monocled cobra) / References: UniProt: P01391 Naja kaouthia (monocled cobra) / References: UniProt: P01391 |
|---|
-Antibody , 1 types, 1 molecules F
| #1: Antibody | Mass: 56329.621 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lama glama (llama) / Production host: Lama glama (llama) / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
|---|
-Sugars , 5 types, 8 molecules 
| #6: Polysaccharide | alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]alpha-D-mannopyranose-(1-6)-[alpha-D- ...alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 1235.105 Da / Num. of mol.: 1 / Mass: 1235.105 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
|---|---|
| #7: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 424.401 Da / Num. of mol.: 1 / Mass: 424.401 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
| #8: Polysaccharide | alpha-D-mannopyranose-(1-3)-alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]beta-D- ...alpha-D-mannopyranose-(1-3)-alpha-D-mannopyranose-(1-6)-[alpha-D-mannopyranose-(1-3)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 1072.964 Da / Num. of mol.: 1 / Mass: 1072.964 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
| #9: Polysaccharide | alpha-D-mannopyranose-(1-6)-beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1- ...alpha-D-mannopyranose-(1-6)-beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 748.682 Da / Num. of mol.: 1 / Mass: 748.682 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
| #10: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine |
-Non-polymers , 3 types, 3 molecules 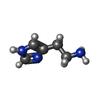



| #11: Chemical | ChemComp-HSM /  Histamine Histamine |
|---|---|
| #12: Chemical | ChemComp-ZN / |
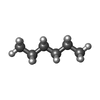
| #13: Chemical | ChemComp-HEX /  Hexane Hexane |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.24 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.25 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||||||||
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: DIFFRACTION / Nominal defocus max: 1800 nm / Nominal defocus min: 600 nm / Nominal defocus max: 1800 nm / Nominal defocus min: 600 nm |
| Image recording | Average exposure time: 2.19 sec. / Electron dose: 50.69 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
3D reconstruction | Resolution: 3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 78662 / Symmetry type: POINT |
 Movie
Movie Controller
Controller













 PDBj
PDBj









