+Search query
-Structure paper
| Title | A panel of nanobodies recognizing conserved hidden clefts of all SARS-CoV-2 spike variants including Omicron. |
|---|---|
| Journal, issue, pages | Commun Biol, Vol. 5, Issue 1, Page 669, Year 2022 |
| Publish date | Jul 6, 2022 |
 Authors Authors | Ryota Maeda / Junso Fujita / Yoshinobu Konishi / Yasuhiro Kazuma / Hiroyuki Yamazaki / Itsuki Anzai / Tokiko Watanabe / Keishi Yamaguchi / Kazuki Kasai / Kayoko Nagata / Yutaro Yamaoka / Kei Miyakawa / Akihide Ryo / Kotaro Shirakawa / Kei Sato / Fumiaki Makino / Yoshiharu Matsuura / Tsuyoshi Inoue / Akihiro Imura / Keiichi Namba / Akifumi Takaori-Kondo /  |
| PubMed Abstract | We are amid the historic coronavirus infectious disease 2019 (COVID-19) pandemic. Imbalances in the accessibility of vaccines, medicines, and diagnostics among countries, regions, and populations, ...We are amid the historic coronavirus infectious disease 2019 (COVID-19) pandemic. Imbalances in the accessibility of vaccines, medicines, and diagnostics among countries, regions, and populations, and those in war crises, have been problematic. Nanobodies are small, stable, customizable, and inexpensive to produce. Herein, we present a panel of nanobodies that can detect the spike proteins of five SARS-CoV-2 variants of concern (VOCs) including Omicron. Here we show via ELISA, lateral flow, kinetic, flow cytometric, microscopy, and Western blotting assays that our nanobodies can quantify the spike variants. This panel of nanobodies broadly neutralizes viral infection caused by pseudotyped and authentic SARS-CoV-2 VOCs. Structural analyses show that the P86 clone targets epitopes that are conserved yet unclassified on the receptor-binding domain (RBD) and contacts the N-terminal domain (NTD). Human antibodies rarely access both regions; consequently, the clone buries hidden crevasses of SARS-CoV-2 spike proteins that go undetected by conventional antibodies. |
 External links External links |  Commun Biol / Commun Biol /  PubMed:35794202 / PubMed:35794202 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 1.6 - 3.29 Å |
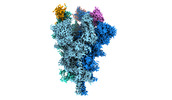
| Structure data | EMDB-32078, PDB-7vq0: 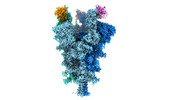 EMDB-32079: Cryo-EM structure of the SARS-CoV-2 spike protein (3-up RBD) bound to neutralizing nanobodies P86 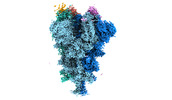 EMDB-32080: Cryo-EM structure of the SARS-CoV-2 spike protein (1-up RBD) bound to neutralizing nanobodies P17 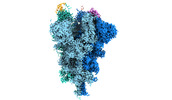 EMDB-32081: Cryo-EM structure of the SARS-CoV-2 spike protein (2-up RBD) bound to neutralizing nanobodies P17  PDB-7vpy: |
| Chemicals |  ChemComp-SO4:  ChemComp-EDO:  ChemComp-HOH:  ChemComp-NAG: |
| Source |
|
 Keywords Keywords |  IMMUNE SYSTEM / IMMUNE SYSTEM /  SARS-CoV-2 / SARS-CoV-2 /  VIRAL PROTEIN/IMMUNE SYSTEM / spike / VIRAL PROTEIN/IMMUNE SYSTEM / spike /  nanobody / VHH / nanobody / VHH /  VIRAL PROTEIN / VIRAL PROTEIN /  VIRAL PROTEIN-IMMUNE SYSTEM complex VIRAL PROTEIN-IMMUNE SYSTEM complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers