+Search query
-Structure paper
| Title | A common mechanism of Sec61 translocon inhibition by small molecules. |
|---|---|
| Journal, issue, pages | Nat Chem Biol, Vol. 19, Issue 9, Page 1063-1071, Year 2023 |
| Publish date | May 11, 2023 |
 Authors Authors | Samuel Itskanov / Laurie Wang / Tina Junne / Rumi Sherriff / Li Xiao / Nicolas Blanchard / Wei Q Shi / Craig Forsyth / Dominic Hoepfner / Martin Spiess / Eunyong Park /    |
| PubMed Abstract | The Sec61 complex forms a protein-conducting channel in the endoplasmic reticulum membrane that is required for secretion of soluble proteins and production of many membrane proteins. Several natural ...The Sec61 complex forms a protein-conducting channel in the endoplasmic reticulum membrane that is required for secretion of soluble proteins and production of many membrane proteins. Several natural and synthetic small molecules specifically inhibit Sec61, generating cellular effects that are useful for therapeutic purposes, but their inhibitory mechanisms remain unclear. Here we present near-atomic-resolution structures of human Sec61 inhibited by a comprehensive panel of structurally distinct small molecules-cotransin, decatransin, apratoxin, ipomoeassin, mycolactone, cyclotriazadisulfonamide and eeyarestatin. All inhibitors bind to a common lipid-exposed pocket formed by the partially open lateral gate and plug domain of Sec61. Mutations conferring resistance to the inhibitors are clustered at this binding pocket. The structures indicate that Sec61 inhibitors stabilize the plug domain in a closed state, thereby preventing the protein-translocation pore from opening. Our study provides the atomic details of Sec61-inhibitor interactions and the structural framework for further pharmacological studies and drug design. |
 External links External links |  Nat Chem Biol / Nat Chem Biol /  PubMed:37169959 PubMed:37169959 |
| Methods | EM (single particle) |
| Resolution | 2.54 - 3.4 Å |
| Structure data | EMDB-27581, PDB-8dnv: EMDB-27582, PDB-8dnw: EMDB-27583, PDB-8dnx: EMDB-27584, PDB-8dny: EMDB-27585, PDB-8dnz: EMDB-27586, PDB-8do0: EMDB-27587, PDB-8do1: EMDB-27588, PDB-8do2: EMDB-27589, PDB-8do3:  EMDB-29608: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by eeyarestatin I  EMDB-29609: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by cotransin  EMDB-29610: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by apratoxin F  EMDB-29611: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) in a partially-open apo state (Class 1) 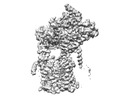 EMDB-29612: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) in a partially-open apo state (Class 2) 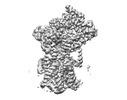 EMDB-29613: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by decatransin 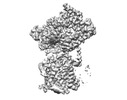 EMDB-29614: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by ipomoeassin F (Class 1) 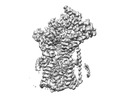 EMDB-29616: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by cyclotriazadisulfonamide (CADA) 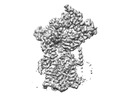 EMDB-29617: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by mycolactone 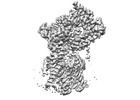 EMDB-29635: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by ipomoeassin F (Class 2) |
| Chemicals |  ChemComp-Q6B:  ChemComp-HOH:  ChemComp-SXF:  ChemComp-SXU: 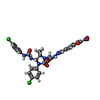 ChemComp-SWR: |
| Source |
|
 Keywords Keywords | PROTEIN TRANSPORT/INHIBITOR /  translocon / translocon /  inhibitor / inhibitor /  protein translocation / protein translocation /  PROTEIN TRANSPORT / PROTEIN TRANSPORT-INHIBITOR complex / decatransin PROTEIN TRANSPORT / PROTEIN TRANSPORT-INHIBITOR complex / decatransin |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers






















 lyngbya bouillonii (bacteria)
lyngbya bouillonii (bacteria)
