[English] 日本語
 Yorodumi
Yorodumi- EMDB-20536: Cryo-EM Structure of the Respiratory Syncytial Virus Polymerase (... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20536 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of the Respiratory Syncytial Virus Polymerase (L) Protein Bound by the Tetrameric Phosphoprotein (P) | |||||||||
 Map data Map data | Sharpened map after non-uniform refinement in cryoSPARC | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  RNA-binding protein / RSV / RNA-binding protein / RSV /  RdRp / RdRp /  RNA-dependent RNA polymerase / PRNTase / polyribonucleotidyl transferase / RNA-dependent RNA polymerase / PRNTase / polyribonucleotidyl transferase /  RNA capping / RNA capping /  viral replication / viral replication /  VIRAL PROTEIN VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationNNS virus cap methyltransferase / GDP polyribonucleotidyltransferase / negative stranded viral RNA replication /  Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides /  viral life cycle / viral life cycle /  virion component / virion component /  : / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / host cell cytoplasm / : / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / host cell cytoplasm /  RNA-directed RNA polymerase ...NNS virus cap methyltransferase / GDP polyribonucleotidyltransferase / negative stranded viral RNA replication / RNA-directed RNA polymerase ...NNS virus cap methyltransferase / GDP polyribonucleotidyltransferase / negative stranded viral RNA replication /  Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides /  viral life cycle / viral life cycle /  virion component / virion component /  : / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / host cell cytoplasm / : / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / host cell cytoplasm /  RNA-directed RNA polymerase / RNA-directed RNA polymerase /  RNA-dependent RNA polymerase activity / RNA-dependent RNA polymerase activity /  GTPase activity / GTPase activity /  ATP binding / ATP binding /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |   Human respiratory syncytial virus A2 Human respiratory syncytial virus A2 | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Gilman MSA / McLellan JS | |||||||||
 Citation Citation |  Journal: Cell / Year: 2019 Journal: Cell / Year: 2019Title: Structure of the Respiratory Syncytial Virus Polymerase Complex. Authors: Morgan S A Gilman / Cheng Liu / Amy Fung / Ishani Behera / Paul Jordan / Peter Rigaux / Nina Ysebaert / Sergey Tcherniuk / Julien Sourimant / Jean-François Eléouët / Priscila Sutto-Ortiz ...Authors: Morgan S A Gilman / Cheng Liu / Amy Fung / Ishani Behera / Paul Jordan / Peter Rigaux / Nina Ysebaert / Sergey Tcherniuk / Julien Sourimant / Jean-François Eléouët / Priscila Sutto-Ortiz / Etienne Decroly / Dirk Roymans / Zhinan Jin / Jason S McLellan /    Abstract: Numerous interventions are in clinical development for respiratory syncytial virus (RSV) infection, including small molecules that target viral transcription and replication. These processes are ...Numerous interventions are in clinical development for respiratory syncytial virus (RSV) infection, including small molecules that target viral transcription and replication. These processes are catalyzed by a complex comprising the RNA-dependent RNA polymerase (L) and the tetrameric phosphoprotein (P). RSV P recruits multiple proteins to the polymerase complex and, with the exception of its oligomerization domain, is thought to be intrinsically disordered. Despite their critical roles in RSV transcription and replication, structures of L and P have remained elusive. Here, we describe the 3.2-Å cryo-EM structure of RSV L bound to tetrameric P. The structure reveals a striking tentacular arrangement of P, with each of the four monomers adopting a distinct conformation. The structure also rationalizes inhibitor escape mutants and mutations observed in live-attenuated vaccine candidates. These results provide a framework for determining the molecular underpinnings of RSV replication and transcription and should facilitate the design of effective RSV inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20536.map.gz emd_20536.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20536-v30.xml emd-20536-v30.xml emd-20536.xml emd-20536.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20536_fsc.xml emd_20536_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_20536.png emd_20536.png | 55.6 KB | ||
| Filedesc metadata |  emd-20536.cif.gz emd-20536.cif.gz | 7 KB | ||
| Others |  emd_20536_additional.map.gz emd_20536_additional.map.gz | 32.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20536 http://ftp.pdbj.org/pub/emdb/structures/EMD-20536 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20536 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20536 | HTTPS FTP |
-Related structure data
| Related structure data |  6pzkMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20536.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20536.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map after non-uniform refinement in cryoSPARC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.075 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
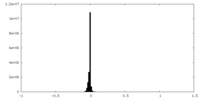
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unsharpened map after non-uniform refinement in cryoSPARC
| File | emd_20536_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map after non-uniform refinement in cryoSPARC | ||||||||||||
| Projections & Slices |
| ||||||||||||
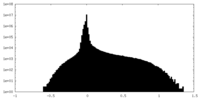
| Density Histograms |
- Sample components
Sample components
-Entire : Respiratory Syncytial Virus Polymerase (L) Protein Bound by the T...
| Entire | Name: Respiratory Syncytial Virus Polymerase (L) Protein Bound by the Tetrameric Phosphoprotein (P) |
|---|---|
| Components |
|
-Supramolecule #1: Respiratory Syncytial Virus Polymerase (L) Protein Bound by the T...
| Supramolecule | Name: Respiratory Syncytial Virus Polymerase (L) Protein Bound by the Tetrameric Phosphoprotein (P) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Human respiratory syncytial virus A2 Human respiratory syncytial virus A2 |
| Molecular weight | Theoretical: 370 KDa |
-Macromolecule #1: RNA-directed RNA polymerase L
| Macromolecule | Name: RNA-directed RNA polymerase L / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number:  RNA-directed RNA polymerase RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:   Human respiratory syncytial virus A2 / Strain: A2 Human respiratory syncytial virus A2 / Strain: A2 |
| Molecular weight | Theoretical: 254.48275 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MGSWSHPQFE KGSGSGSSWS HPQFEKGSGS LVPRGSMDPI INGNSANVYL TDSYLKGVIS FSECNALGSY IFNGPYLKND YTNLISRQN PLIEHMNLKK LNITQSLISK YHKGEIKLEE PTYFQSLLMT YKSMTSSEQI ATTNLLKKII RRAIEISDVK V YAILNKLG ...String: MGSWSHPQFE KGSGSGSSWS HPQFEKGSGS LVPRGSMDPI INGNSANVYL TDSYLKGVIS FSECNALGSY IFNGPYLKND YTNLISRQN PLIEHMNLKK LNITQSLISK YHKGEIKLEE PTYFQSLLMT YKSMTSSEQI ATTNLLKKII RRAIEISDVK V YAILNKLG LKEKDKIKSN NGQDEDNSVI TTIIKDDILS AVKDNQSHLK ADKNHSTKQK DTIKTTLLKK LMCSMQHPPS WL IHWFNLY TKLNNILTQY RSNEVKNHGF TLIDNQTLSG FQFILNQYGC IVYHKELKRI TVTTYNQFLT WKDISLSRLN VCL ITWISN CLNTLNKSLG LRCGFNNVIL TQLFLYGDCI LKLFHNEGFY IIKEVEGFIM SLILNITEED QFRKRFYNSM LNNI TDAAN KAQKNLLSRV CHTLLDKTVS DNIINGRWII LLSKFLKLIK LAGDNNLNNL SELYFLFRIF GHPMVDERQA MDAVK INCN ETKFYLLSSL SMLRGAFIYR IIKGFVNNYN RWPTLRNAIV LPLRWLTYYK LNTYPSLLEL TERDLIVLSG LRFYRE FRL PKKVDLEMII NDKAISPPKN LIWTSFPRNY MPSHIQNYIE HEKLKFSESD KSRRVLEYYL RDNKFNECDL YNCVVNQ SY LNNPNHVVSL TGKERELSVG RMFAMQPGMF RQVQILAEKM IAENILQFFP ESLTRYGDLE LQKILELKAG ISNKSNRY N DNYNNYISKC SIITDLSKFN QAFRYETSCI CSDVLDELHG VQSLFSWLHL TIPHVTIICT YRHAPPYIGD HIVDLNNVD EQSGLYRYHM GGIEGWCQKL WTIEAISLLD LISLKGKFSI TALINGDNQS IDISKPIRLM EGQTHAQADY LLALNSLKLL YKEYAGIGH KLKGTETYIS RDMQFMSKTI QHNGVYYPAS IKKVLRVGPW INTILDDFKV SLESIGSLTQ ELEYRGESLL C SLIFRNVW LYNQIALQLK NHALCNNKLY LDILKVLKHL KTFFNLDNID TALTLYMNLP MLFGGGDPNL LYRSFYRRTP DF LTEAIVH SVFILSYYTN HDLKDKLQDL SDDRLNKFLT CIITFDKNPN AEFVTLMRDP QALGSERQAK ITSEINRLAV TEV LSTAPN KIFSKSAQHY TTTEIDLNDI MQNIEPTYPH GLRVVYESLP FYKAEKIVNL ISGTKSITNI LEKTSAIDLT DIDR ATEMM RKNITLLIRI LPLDCNRDKR EILSMENLSI TELSKYVRER SWSLSNIVGV TSPSIMYTMD IKYTTSTISS GIIIE KYNV NSLTRGERGP TKPWVGSSTQ EKKTMPVYNR QVLTKKQRDQ IDLLAKLDWV YASIDNKDEF MEELSIGTLG LTYEKA KKL FPQYLSVNYL HRLTVSSRPC EFPASIPAYR TTNYHFDTSP INRILTEKYG DEDIDIVFQN CISFGLSLMS VVEQFTN VC PNRIILIPKL NEIHLMKPPI FTGDVDIHKL KQVIQKQHMF LPDKISLTQY VELFLSNKTL KSGSHVNSNL ILAHKISD Y FHNTYILSTN LAGHWILIIQ LMKDSKGIFE KDWGEGYITD HMFINLKVFF NAYKTYLLCF HKGYGKAKLE CDMNTSDLL CVLELIDSSY WKSMSKVFLE QKVIKYILSQ DASLHRVKGC HSFKLWFLKR LNVAEFTVCP WVVNIDYHPT HMKAILTYID LVRMGLINI DRIHIKNKHK FNDEFYTSNL FYINYNFSDN THLLTKHIRI ANSELENNYN KLYHPTPETL ENILANPIKS N DKKTLNDY CIGKNVDSIM LPLLSNKKLI KSSAMIRTNY SKQDLYNLFP MVVIDRIIDH SGNTAKSNQL YTTTSHQISL VH NSTSLYC MLPWHHINRF NFVFSSTGCK ISIEYILKDL KIKDPNCIAF IGEGAGNLLL RTVVELHPDI RYIYRSLKDC NDH SLPIEF LRLYNGHINI DYGENLTIPA TDATNNIHWS YLHIKFAEPI SLFVCDAELS VTVNWSKIII EWSKHVRKCK YCSS VNKCM LIVKYHAQDD IDFKLDNITI LKTYVCLGSK LKGSEVYLVL TIGPANIFPV FNVVQNAKLI LSRTKNFIMP KKADK ESID ANIKSLIPFL CYPITKKGIN TALSKLKSVV SGDILSYSIA GRNEVFSNKL INHKHMNILK WFNHVLNFRS TELNYN HLY MVESTYPYLS ELLNSLTTNE LKKLIKITGS LLYNFHNE UniProtKB: RNA-directed RNA polymerase L |
-Macromolecule #2: Phosphoprotein
| Macromolecule | Name: Phosphoprotein / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human respiratory syncytial virus A2 / Strain: A2 Human respiratory syncytial virus A2 / Strain: A2 |
| Molecular weight | Theoretical: 29.062895 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MEKFAPEFHG EDANNRATKF LESIKGKFTS PKDPKKKDSI ISVNSIDIEV TKESPITSNS TIINPTNETD DTAGNKPNYQ RKPLVSFKE DPTPSDNPFS KLYKETIETF DNNEEESSYS YEEINDQTND NITARLDRID EKLSEILGML HTLVVASAGP T SARDGIRD ...String: MEKFAPEFHG EDANNRATKF LESIKGKFTS PKDPKKKDSI ISVNSIDIEV TKESPITSNS TIINPTNETD DTAGNKPNYQ RKPLVSFKE DPTPSDNPFS KLYKETIETF DNNEEESSYS YEEINDQTND NITARLDRID EKLSEILGML HTLVVASAGP T SARDGIRD AMIGLREEMI EKIRTEALMT NDRLEAMARL RNEESEKMAK DTSDEVSLNP TSEKLNNLLE GNDSDNDLSL ED FKGENKY FQGHHHHHH UniProtKB:  Phosphoprotein Phosphoprotein |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.29 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Material: GOLD | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 48.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X