+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1quz | ||||||
|---|---|---|---|---|---|---|---|
| Title | Solution structure of the potassium channel scorpion toxin HSTX1 | ||||||
 Components Components | HSTX1 TOXIN | ||||||
 Keywords Keywords |  TOXIN / TOXIN /  SCORPION TOXIN / ALPHA BETA / MOLECULAR MODELING / SCORPION TOXIN / ALPHA BETA / MOLECULAR MODELING /  POTASSIUM CHANNEL POTASSIUM CHANNEL | ||||||
| Function / homology | Scorpion short toxins signature. / Scorpion short chain toxin, potassium channel inhibitor / Scorpion short toxin, BmKK2 /  Knottin, scorpion toxin-like superfamily / ion channel inhibitor activity / potassium channel regulator activity / Knottin, scorpion toxin-like superfamily / ion channel inhibitor activity / potassium channel regulator activity /  toxin activity / extracellular region / Potassium channel toxin alpha-KTx 6.3 toxin activity / extracellular region / Potassium channel toxin alpha-KTx 6.3 Function and homology information Function and homology information | ||||||
| Method |  SOLUTION NMR / A COMPLETLY EXTENDED CONFORMATION WAS USED AS THE STARTING STRUCTURE. 25 ITERATIONS WERE MADE. 500 STRUCTURES WERE CALCULATED. RESTRAINED MOLECULAR DYNAMICS AT 600K A SLOW COOLING. MINIMIZATION WERE MADE WITH AN ELECTROSTATIC TERM TOPALLH22X.PRO, PARALLH22X.PRO SOLUTION NMR / A COMPLETLY EXTENDED CONFORMATION WAS USED AS THE STARTING STRUCTURE. 25 ITERATIONS WERE MADE. 500 STRUCTURES WERE CALCULATED. RESTRAINED MOLECULAR DYNAMICS AT 600K A SLOW COOLING. MINIMIZATION WERE MADE WITH AN ELECTROSTATIC TERM TOPALLH22X.PRO, PARALLH22X.PRO | ||||||
 Authors Authors | Savarin, P. / Romi-Lebrun, R. / Zinn-Justin, S. / Lebrun, B. / Nakajima, T. / Gilquin, B. / Menez, A. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 1999 Journal: Protein Sci. / Year: 1999Title: Structural and functional consequences of the presence of a fourth disulfide bridge in the scorpion short toxins: solution structure of the potassium channel inhibitor HsTX1. Authors: Savarin, P. / Romi-Lebrun, R. / Zinn-Justin, S. / Lebrun, B. / Nakajima, T. / Gilquin, B. / Menez, A. #1:  Journal: Biochem.J. / Year: 1997 Journal: Biochem.J. / Year: 1997Title: A four-disulfide-bridged toxin, with high affinity towards voltage-gatedc K+ channels, isolated from Heterometrus spinnifer (scorpionidae) venom Authors: Lebrun, B. / Romi-Lebrun, R. / Martin-Eauclaire, M.F. / Yasuda, A. / Ishiguro, M. / Oyama, Y. / Pongs, O. / Nakajima, T. #2:  Journal: Toxicon / Year: 1998 Journal: Toxicon / Year: 1998Title: Maurotoxin, a four disulfide bridges scorpion toxin acting on K+ channels. Authors: Rochat, H. / Kharrat, R. / Sabatier, J.M. / Mansuelle, P. / Crest, M. / Martin-Eauclaire, M.F. / Sampieri, F. / Oughideni, R. / Mabrouk, K. / Jacquet, G. / Van Rietschoten, J. / El Ayeb, M. #3:  Journal: Biochemistry / Year: 1997 Journal: Biochemistry / Year: 1997Title: Purification, characterization, and synthesis of three novel toxins from the Chinese scorpion Buthus martensi, which act on K+ channels Authors: Romi-Lebrun, R. / Lebrun, B. / Martin-Eauclaire, M.F. / Ishiguro, M. / Escoubas, P. / Wu, F.Q. / Hisada, M. / Pongs, O. / Nakajima, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1quz.cif.gz 1quz.cif.gz | 202.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1quz.ent.gz pdb1quz.ent.gz | 175.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1quz.json.gz 1quz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qu/1quz https://data.pdbj.org/pub/pdb/validation_reports/qu/1quz ftp://data.pdbj.org/pub/pdb/validation_reports/qu/1quz ftp://data.pdbj.org/pub/pdb/validation_reports/qu/1quz | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 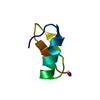
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 3834.593 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: SOLID PHASE CHEMICAL SYNTHESIS USING THE FMOC METHODOLOGY. THE SEQUENCE OF THIS PEPTIDE OCCURS NATURALLY IN SCORPIONS (SCORPIONIDAE). References: UniProt: P59867 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details |
| ||||||
|---|---|---|---|---|---|---|---|
| Sample conditions | pH: 4.0 / Pressure: 1 atm / Temperature: 308 K | ||||||
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer | Type: Bruker DRX / Manufacturer: Bruker / Model : DRX / Field strength: 500 MHz : DRX / Field strength: 500 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: A COMPLETLY EXTENDED CONFORMATION WAS USED AS THE STARTING STRUCTURE. 25 ITERATIONS WERE MADE. 500 STRUCTURES WERE CALCULATED. RESTRAINED MOLECULAR DYNAMICS AT 600K A SLOW COOLING. ...Method: A COMPLETLY EXTENDED CONFORMATION WAS USED AS THE STARTING STRUCTURE. 25 ITERATIONS WERE MADE. 500 STRUCTURES WERE CALCULATED. RESTRAINED MOLECULAR DYNAMICS AT 600K A SLOW COOLING. MINIMIZATION WERE MADE WITH AN ELECTROSTATIC TERM TOPALLH22X.PRO, PARALLH22X.PRO Software ordinal: 1 | ||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||
| NMR ensemble | Conformer selection criteria: all calculated structures submitted Conformers calculated total number: 20 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller










 PDBj
PDBj

