[English] 日本語
 Yorodumi
Yorodumi- PDB-9s07: 3 us Intermediate-state of CBD of TtCarH at 30 mJ/cm2 laser fluen... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9s07 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
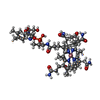
| Title | 3 us Intermediate-state of CBD of TtCarH at 30 mJ/cm2 laser fluence (TR-SFX, SwissFEL), refined against extrapolated structure factors | ||||||||||||
 Components Components | Probable transcriptional regulator | ||||||||||||
 Keywords Keywords | DNA BINDING PROTEIN / SFX / Room temperature / serial crystallography / CarH / photoreceptor / vitamin B12 / cobalamin / coenzyme B12 / time-resolved crystallography | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcobalamin binding / DNA-binding transcription factor activity / DNA binding / metal ion binding / identical protein binding Similarity search - Function | ||||||||||||
| Biological species |   Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / FREE ELECTRON LASER /  MOLECULAR REPLACEMENT / Resolution: 2.6 Å MOLECULAR REPLACEMENT / Resolution: 2.6 Å | ||||||||||||
 Authors Authors | Rios-Santacruz, R. / Coquelle, N. / Colletier, J.P. / De Zitter, E. / Schiro, G. / Weik, M. | ||||||||||||
| Funding support |  France, 3items France, 3items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2026 Journal: Nature / Year: 2026Title: Integrated structural dynamics uncover new modes of B12 photoreceptor activation Authors: Rios-Santacruz, R. / Coquelle, N. / Colletier, J.P. / De Zitter, E. / Schiro, G. / Weik, M. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9s07.cif.gz 9s07.cif.gz | 175.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9s07.ent.gz pdb9s07.ent.gz | 138.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9s07.json.gz 9s07.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/s0/9s07 https://data.pdbj.org/pub/pdb/validation_reports/s0/9s07 ftp://data.pdbj.org/pub/pdb/validation_reports/s0/9s07 ftp://data.pdbj.org/pub/pdb/validation_reports/s0/9s07 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  9s06C  9s08C  9s09C  9s0aC  9s0bC  9s0cC  9s0dC  9s0eC  9s0fC  9s0gC  9s0hC  9s0iC  9s0jC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23259.801 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Thermus thermophilus (bacteria) / Gene: TT_P0056 / Plasmid: pET21b / Production host: Thermus thermophilus (bacteria) / Gene: TT_P0056 / Plasmid: pET21b / Production host:  #2: Chemical | ChemComp-5AD / #3: Chemical | ChemComp-B12 / #4: Water | ChemComp-HOH / | Has ligand of interest | Y | Has protein modification | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.66 Å3/Da / Density % sol: 53.77 % Description: plate-like microcrystals with a size around 15umx5umx2um |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: batch mode / pH: 7.5 / Details: 0.1 M HEPES pH 7.5, 20% PEG 10000. |
-Data collection
| Diffraction | Mean temperature: 293.15 K / Serial crystal experiment: Y |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: SwissFEL ARAMIS FREE ELECTRON LASER / Site: SwissFEL ARAMIS  / Beamline: ESC / Wavelength: 1.13 Å / Beamline: ESC / Wavelength: 1.13 Å |
| Detector | Type: PSI JUNGFRAU 8M / Detector: PIXEL / Date: Jun 7, 2024 / Frequency: 100 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.13 Å / Relative weight: 1 |
| Reflection | Resolution: 2.6→50 Å / Num. obs: 31460 / % possible obs: 100 % / Redundancy: 501 % / Biso Wilson estimate: 51.64 Å2 / CC1/2: 0.986 / CC star: 0.997 / R split: 0.136 / Net I/σ(I): 5.9 |
| Reflection shell | Resolution: 2.6→2.65 Å / Redundancy: 349 % / Mean I/σ(I) obs: 0.8 / Num. unique obs: 2056 / CC1/2: 0.541 / CC star: 0.838 / R split: 1.034 / % possible all: 100 |
| Serial crystallography measurement | Focal spot size: 15.54 µm2 / Pulse duration: 35 fsec. / Pulse energy: 280 µJ / Pulse photon energy: 11 keV / XFEL pulse repetition rate: 100 Hz |
| Serial crystallography sample delivery | Description: Fixed target / Method: fixed target |
| Serial crystallography sample delivery fixed target | Description: micro-structured polymer fixed targets / Sample dehydration prevention: mylar film / Sample holding: mesh |
| Serial crystallography data reduction | Crystal hits: 44725 / Frames total: 65740 / Lattices indexed: 33798 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.6→19.96 Å / SU ML: 0.48 / Cross valid method: FREE R-VALUE / Phase error: 39.98 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 2.6→19.96 Å / SU ML: 0.48 / Cross valid method: FREE R-VALUE / Phase error: 39.98 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→19.96 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj