[English] 日本語
 Yorodumi
Yorodumi- PDB-9pct: Structure of Porcine Trypsin Crystals Grown from PEG Complexed wi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9pct | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of Porcine Trypsin Crystals Grown from PEG Complexed with Crystallization Additives III | ||||||
 Components Components | Trypsin | ||||||
 Keywords Keywords | PEPTIDE BINDING PROTEIN / sucrose / crystallization / additives / Silver Bullets / organic acids / ligands | ||||||
| Function / homology |  Function and homology information Function and homology informationtrypsin / digestion / serine-type endopeptidase activity / proteolysis / extracellular space / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.41 Å MOLECULAR REPLACEMENT / Resolution: 1.41 Å | ||||||
 Authors Authors | McPherson, A. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structure of Porcine Trypsin Crystals Grown from PEG Complexed with Crystallization Additives III Authors: McPherson, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9pct.cif.gz 9pct.cif.gz | 197.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9pct.ent.gz pdb9pct.ent.gz | 133 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9pct.json.gz 9pct.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9pct_validation.pdf.gz 9pct_validation.pdf.gz | 2.9 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9pct_full_validation.pdf.gz 9pct_full_validation.pdf.gz | 3 MB | Display | |
| Data in XML |  9pct_validation.xml.gz 9pct_validation.xml.gz | 20.4 KB | Display | |
| Data in CIF |  9pct_validation.cif.gz 9pct_validation.cif.gz | 26.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pc/9pct https://data.pdbj.org/pub/pdb/validation_reports/pc/9pct ftp://data.pdbj.org/pub/pdb/validation_reports/pc/9pct ftp://data.pdbj.org/pub/pdb/validation_reports/pc/9pct | HTTPS FTP |
-Related structure data
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 24428.424 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Sugars , 2 types, 2 molecules
| #2: Polysaccharide | beta-D-fructofuranose-(2-1)-alpha-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|---|
| #3: Polysaccharide | alpha-D-glucopyranose-(1-2)-beta-D-fructofuranose Type: oligosaccharide / Mass: 342.297 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source |
-Non-polymers , 14 types, 190 molecules 





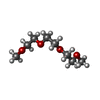

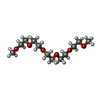



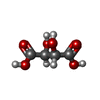














| #4: Chemical | ChemComp-CA / | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #5: Chemical | | #6: Chemical | #7: Chemical | #8: Chemical | ChemComp-PEG / #9: Chemical | #10: Chemical | ChemComp-PG5 / #11: Chemical | #12: Chemical | #13: Chemical | #14: Chemical | #15: Chemical | ChemComp-PO4 / | #16: Chemical | ChemComp-TAR / | #17: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 48.66 % / Description: rectangular prisms |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, sitting drop Details: Sitting drop vapor diffusion in Cryschem plates with 0.6 ml reservoirs of 30% PEG 3350 buffered at pH 6.5 with 0.1 M HEPES. Drops 3 ul of reservoir, 2 ul of additive mix ( TACSIMATE, ...Details: Sitting drop vapor diffusion in Cryschem plates with 0.6 ml reservoirs of 30% PEG 3350 buffered at pH 6.5 with 0.1 M HEPES. Drops 3 ul of reservoir, 2 ul of additive mix ( TACSIMATE, glycerol, sucrose, sorbitol), 3 ul of 40 mg/ml stock protein solution buffered at pH 6.5 with 0.1 M HEPES. |
-Data collection
| Diffraction | Mean temperature: 295 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU / Wavelength: 1.54 Å ROTATING ANODE / Type: RIGAKU / Wavelength: 1.54 Å |
| Detector | Type: RIGAKU / Detector: IMAGE PLATE / Date: Jun 12, 2012 / Details: Osmic mirrors |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54 Å / Relative weight: 1 |
| Reflection | Resolution: 1.41→27.1 Å / Num. obs: 36756 / % possible obs: 78.7 % / Redundancy: 3.41 % / Biso Wilson estimate: 18.65 Å2 / Rmerge(I) obs: 0.053 / Net I/σ(I): 13.5 |
| Reflection shell | Resolution: 1.41→1.46 Å / Rmerge(I) obs: 0.27 / Mean I/σ(I) obs: 2 / Num. unique obs: 800 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.41→26.31 Å / SU ML: 0.1247 / Cross valid method: FREE R-VALUE / σ(F): 1 / Phase error: 18.0863 MOLECULAR REPLACEMENT / Resolution: 1.41→26.31 Å / SU ML: 0.1247 / Cross valid method: FREE R-VALUE / σ(F): 1 / Phase error: 18.0863 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 Details: This structure is reported from an X-ray analysis of crystals grown with 30% PEG as the precipitating agent and was refined using REFINE from the PHENIX system. It was assumed in this ...Details: This structure is reported from an X-ray analysis of crystals grown with 30% PEG as the precipitating agent and was refined using REFINE from the PHENIX system. It was assumed in this refinement and thought appropriate to treat the solvent regions as mixtures or amalgams of disordered water molecules, ordered molecules of water, and PEG molecules of short, different lengths. The inclusion of a large number of PEG molecules is consistent with data indicating that PEG constitutes a large volume of the non protein occupied crystal, with previous reports in the literature of PEG constituents of the solvent regions, and with the strand like appearance of the low level electron density in the solvent regions. Because all of the PEG molecules included here are of low to moderate occupancy, and the clash analysis neglects this property, the clash score reported by REFINE and by the PDB in the validation report is erroneous and should be disregarded. It is not a valid representation of reality. In addition, automated approaches to placing water molecules in crystals grown from PEG, are not to be trusted. If the PEG molecules are omitted from the model and only the protein and ligands are included then the clash score is in the low single digits.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 43.32 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.41→26.31 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj
























