+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9o2a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Heparanase P6 in complex with fragment J69 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE / Heparanase / small molecule / cancer / complex / therapeutics | |||||||||
| Function / homology |  Function and homology information Function and homology informationheparanase / heparanase activity / regulation of hair follicle development / heparin proteoglycan metabolic process / heparan sulfate proteoglycan catabolic process / beta-glucuronidase activity / HS-GAG degradation / positive regulation of hair follicle development / syndecan binding / proteoglycan metabolic process ...heparanase / heparanase activity / regulation of hair follicle development / heparin proteoglycan metabolic process / heparan sulfate proteoglycan catabolic process / beta-glucuronidase activity / HS-GAG degradation / positive regulation of hair follicle development / syndecan binding / proteoglycan metabolic process / vascular wound healing / protein transmembrane transport / establishment of endothelial barrier / angiogenesis involved in wound healing / positive regulation of osteoblast proliferation / positive regulation of vascular endothelial growth factor production / positive regulation of blood coagulation / lysosomal lumen / cell-matrix adhesion / extracellular matrix / specific granule lumen / lysosome / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / membrane raft / intracellular membrane-bounded organelle / lysosomal membrane / response to antibiotic / Neutrophil degranulation / extracellular space / extracellular region / nucleoplasm / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | |||||||||
 Authors Authors | Davies, L.J. / Frkic, R.L. / Jackson, C.J. | |||||||||
| Funding support |  Australia, 2items Australia, 2items
| |||||||||
 Citation Citation |  Journal: Acs Med.Chem.Lett. / Year: 2026 Journal: Acs Med.Chem.Lett. / Year: 2026Title: Fragment Screening and Structure-Guided Development of Heparanase Inhibitors Reveal Orthosteric and Allosteric Inhibition Authors: Davies, L.J. / Whitefield, C. / Kim, H. / Nitsche, C. / Jackson, C.J. / Frkic, R.L. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9o2a.cif.gz 9o2a.cif.gz | 108.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9o2a.ent.gz pdb9o2a.ent.gz | 79.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9o2a.json.gz 9o2a.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/o2/9o2a https://data.pdbj.org/pub/pdb/validation_reports/o2/9o2a ftp://data.pdbj.org/pub/pdb/validation_reports/o2/9o2a ftp://data.pdbj.org/pub/pdb/validation_reports/o2/9o2a | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  9o1rC  9o1sC  9o1tC  9o1uC  9o1vC  9o1wC  9o1xC  9o1yC  9o1zC  9o20C  9o21C  9o22C  9o23C  9o24C  9o25C  9o26C  9o27C  9o28C  9o29C  9o2bC  9o2cC  9o2dC  9o2eC  9o2fC  9o2gC  9o2hC  9o2iC  9o2jC  9o2kC  9o2lC  9o2mC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 42986.309 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HPSE, HEP, HPA, HPA1, HPR1, HPSE1, HSE1 / Production host: Homo sapiens (human) / Gene: HPSE, HEP, HPA, HPA1, HPR1, HPSE1, HSE1 / Production host:  |
|---|---|
| #2: Protein | Mass: 8273.514 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HPSE, HEP, HPA, HPA1, HPR1, HPSE1, HSE1 / Production host: Homo sapiens (human) / Gene: HPSE, HEP, HPA, HPA1, HPR1, HPSE1, HSE1 / Production host:  |
-Non-polymers , 4 types, 122 molecules 
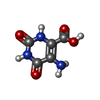





| #3: Chemical | ChemComp-EDO / | ||
|---|---|---|---|
| #4: Chemical | ChemComp-IJZ / | ||
| #5: Chemical | | #6: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.65 Å3/Da / Density % sol: 53.54 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop Details: 0.2 M ammonium sulfate, 0.1 M ammonium acetate, 25% PEG4000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Australian Synchrotron Australian Synchrotron  / Beamline: MX2 / Wavelength: 0.9537 Å / Beamline: MX2 / Wavelength: 0.9537 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Jul 22, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9537 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→42.54 Å / Num. obs: 26851 / % possible obs: 83.4 % / Redundancy: 5.2 % / CC1/2: 0.987 / Rmerge(I) obs: 0.147 / Rpim(I) all: 0.071 / Rrim(I) all: 0.164 / Χ2: 1.03 / Net I/σ(I): 8.2 / Num. measured all: 140773 |
| Reflection shell | Resolution: 2.1→2.16 Å / % possible obs: 84.6 % / Redundancy: 5 % / Rmerge(I) obs: 1.136 / Num. measured all: 11203 / Num. unique obs: 2219 / CC1/2: 0.596 / Rpim(I) all: 0.551 / Rrim(I) all: 1.27 / Χ2: 0.99 / Net I/σ(I) obs: 1.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.1→42.54 Å / SU ML: 0.29 / Cross valid method: NONE / σ(F): 1.33 / Phase error: 28.73 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 2.1→42.54 Å / SU ML: 0.29 / Cross valid method: NONE / σ(F): 1.33 / Phase error: 28.73 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→42.54 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj



