+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9k24 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of alpha-synuclein Mini P fibril | ||||||
 Components Components | Alpha-synuclein | ||||||
 Keywords Keywords | PROTEIN FIBRIL / amyloid | ||||||
| Function / homology |  Function and homology information Function and homology informationPKR-mediated signaling / regulation of neurotransmitter secretion / platelet alpha granule membrane / membrane organization / synaptic transmission, dopaminergic / neurotransmitter secretion / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake ...PKR-mediated signaling / regulation of neurotransmitter secretion / platelet alpha granule membrane / membrane organization / synaptic transmission, dopaminergic / neurotransmitter secretion / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / response to desipramine / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / negative regulation of chaperone-mediated autophagy / mitochondrial membrane organization / arachidonate binding / regulation of reactive oxygen species metabolic process / positive regulation of protein localization to cell periphery / negative regulation of platelet-derived growth factor receptor signaling pathway / negative regulation of exocytosis / regulation of glutamate secretion / dopamine biosynthetic process / response to iron(II) ion / negative regulation of thrombin-activated receptor signaling pathway / SNARE complex assembly / positive regulation of neurotransmitter secretion / negative regulation of dopamine metabolic process / regulation of macrophage activation / positive regulation of inositol phosphate biosynthetic process / regulation of locomotion / negative regulation of microtubule polymerization / synaptic vesicle transport / synaptic vesicle priming / transporter regulator activity / dopamine metabolic process / regulation of dopamine secretion / mitochondrial ATP synthesis coupled electron transport / positive regulation of receptor recycling / dynein complex binding / protein complex oligomerization / cuprous ion binding / nuclear outer membrane / response to magnesium ion / positive regulation of exocytosis / positive regulation of endocytosis / regulation of neuronal synaptic plasticity / synaptic vesicle endocytosis / kinesin binding / cysteine-type endopeptidase inhibitor activity / negative regulation of serotonin uptake / response to type II interferon / regulation of presynapse assembly / positive regulation of synaptic transmission / alpha-tubulin binding / beta-tubulin binding / phospholipase binding / behavioral response to cocaine / cellular response to fibroblast growth factor stimulus / phospholipid metabolic process / inclusion body / Hsp70 protein binding / axon terminus / response to interleukin-1 / regulation of microtubule cytoskeleton organization / cellular response to copper ion / positive regulation of release of sequestered calcium ion into cytosol / SNARE binding / adult locomotory behavior / excitatory postsynaptic potential / protein tetramerization / phosphoprotein binding / microglial cell activation / ferrous iron binding / fatty acid metabolic process / synapse organization / regulation of long-term neuronal synaptic plasticity / phospholipid binding / receptor internalization / protein destabilization / tau protein binding / terminal bouton / positive regulation of inflammatory response / long-term synaptic potentiation / synaptic vesicle membrane / actin cytoskeleton / actin binding / growth cone / cellular response to oxidative stress / neuron apoptotic process / cell cortex / histone binding / response to lipopolysaccharide / microtubule binding / chemical synaptic transmission / negative regulation of neuron apoptotic process / cytoskeleton / mitochondrial outer membrane / oxidoreductase activity / postsynapse Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / helical reconstruction / cryo EM / Resolution: 3.6 Å | ||||||
 Authors Authors | Xia, W.C. / Liu, C. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Neuron / Year: 2025 Journal: Neuron / Year: 2025Title: Fibril fuzzy coat is important for α-synuclein pathological transmission activity. Authors: Yuliang Han / Juan Li / Wencheng Xia / Qintong Li / Zihan Sun / Wen Zeng / Yingxin Hu / Kelvin C Luk / Cong Liu / ShengQi Xiang / Zhuohao He /   Abstract: α-synuclein transmission and propagation are hallmarks of synucleinopathies, yet the molecular mechanisms remain elusive. Using α-synuclein preformed fibrils as pathological seeds, we observed a ...α-synuclein transmission and propagation are hallmarks of synucleinopathies, yet the molecular mechanisms remain elusive. Using α-synuclein preformed fibrils as pathological seeds, we observed a gradual decline in neuronal transmission activity during serial propagation. Fibril polymorphisms were identified from the initial generation: mini-P, with higher neuronal seeding activity, and mini-S, which accelerated recombinant α-synuclein aggregation. Changes in their proportions during propagation explained the overall decline in transmission activity. Cryoelectron microscopy and solid-state nuclear magnetic resonance revealed that both fibrils shared similar core regions but differed in their fuzzy coat flexibilities. The interaction between the fuzzy coat and fibril core substantially influenced neuronal transmission, a model further supported by hydrogen/deuterium exchange mass spectrometry. A mini-P-selective antibody identified active fibril types in newly propagated brain regions in human synucleinopathies. This study highlights the fuzzy coat's pivotal role in pathological protein transmission and suggests it as a potential therapeutic target for synucleinopathies. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9k24.cif.gz 9k24.cif.gz | 72.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9k24.ent.gz pdb9k24.ent.gz | 52.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9k24.json.gz 9k24.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k2/9k24 https://data.pdbj.org/pub/pdb/validation_reports/k2/9k24 ftp://data.pdbj.org/pub/pdb/validation_reports/k2/9k24 ftp://data.pdbj.org/pub/pdb/validation_reports/k2/9k24 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  61989MC  9k23C  9lonC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 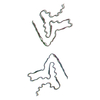
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 14501.185 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Has protein modification | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: FILAMENT / 3D reconstruction method: helical reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cryo-EM structure of alpha-synuclein Mini P fibril / Type: ORGANELLE OR CELLULAR COMPONENT / Entity ID: all / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 55 e/Å2 / Film or detector model: GATAN K3 BIOCONTINUUM (6k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| Helical symmerty | Angular rotation/subunit: 179.59 ° / Axial rise/subunit: 2.4 Å / Axial symmetry: C1 | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 43228 / Symmetry type: HELICAL | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





 PDBj
PDBj
