[English] 日本語
 Yorodumi
Yorodumi- PDB-9h8t: Cryo-EM structure of the atovaquone-inhibited Complex III from th... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9h8t | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the atovaquone-inhibited Complex III from the Chlorocebus sabaeus respirasome | |||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||
 Keywords Keywords | ELECTRON TRANSPORT / complex III / respirasome / mitochondria / green african monkey / atovaquone | |||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationsubthalamus development / pons development / cerebellar Purkinje cell layer development / mitochondrial respiratory chain complex III assembly / pyramidal neuron development / thalamus development / respiratory chain complex / respiratory chain complex III / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity ...subthalamus development / pons development / cerebellar Purkinje cell layer development / mitochondrial respiratory chain complex III assembly / pyramidal neuron development / thalamus development / respiratory chain complex / respiratory chain complex III / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / mitochondrial electron transport, ubiquinol to cytochrome c / hypothalamus development / midbrain development / respiratory electron transport chain / hippocampus development / metalloendopeptidase activity / 2 iron, 2 sulfur cluster binding / electron transfer activity / oxidoreductase activity / mitochondrial inner membrane / heme binding / proteolysis / nucleoplasm / metal ion binding Similarity search - Function | |||||||||||||||||||||||||||
| Biological species |  Chlorocebus sabaeus (green monkey) Chlorocebus sabaeus (green monkey) | |||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.67 Å | |||||||||||||||||||||||||||
 Authors Authors | Maclean, A. / Muhleip, A. | |||||||||||||||||||||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: Structure, assembly and inhibition of the Toxoplasma gondii respiratory chain supercomplex. Authors: Andrew E MacLean / Shikha Shikha / Mariana Ferreira Silva / Max J Gramelspacher / Aaron Nilsen / Katherine M Liebman / Sovitj Pou / Rolf W Winter / Amit Meir / Michael K Riscoe / J Stone ...Authors: Andrew E MacLean / Shikha Shikha / Mariana Ferreira Silva / Max J Gramelspacher / Aaron Nilsen / Katherine M Liebman / Sovitj Pou / Rolf W Winter / Amit Meir / Michael K Riscoe / J Stone Doggett / Lilach Sheiner / Alexander Mühleip /    Abstract: The apicomplexan mitochondrial electron transport chain is essential for parasite survival and displays a divergent subunit composition. Here we report cryo-electron microscopy structures of an ...The apicomplexan mitochondrial electron transport chain is essential for parasite survival and displays a divergent subunit composition. Here we report cryo-electron microscopy structures of an apicomplexan III-IV supercomplex and of the drug target complex III. The supercomplex structure reveals how clade-specific subunits form an apicomplexan-conserved III-IV interface with a unique, kinked architecture, suggesting that supercomplexes evolved independently in different eukaryotic lineages. A knockout resulting in supercomplex disassembly challenges the proposed role of III-IV in electron transfer efficiency as suggested for mammals. Nevertheless, knockout analysis indicates that III-IV is critical for parasite fitness. The complexes from the model parasite Toxoplasma gondii were inhibited with the antimalarial atovaquone, revealing interactions underpinning species specificity. They were also inhibited with endochin-like quinolone (ELQ)-300, an inhibitor in late-stage preclinical development. Notably, in the apicomplexan binding site, ELQ-300 is flipped compared with related compounds in the mammalian enzyme. On the basis of the binding modes and parasite-specific interactions discovered, we designed more potent ELQs with subnanomolar activity against T. gondii. Our findings reveal critical evolutionary differences in the role of supercomplexes in mitochondrial biology and provide insight into cytochrome b inhibition, informing future drug discovery. | |||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9h8t.cif.gz 9h8t.cif.gz | 1.5 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9h8t.ent.gz pdb9h8t.ent.gz | 1.3 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9h8t.json.gz 9h8t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h8/9h8t https://data.pdbj.org/pub/pdb/validation_reports/h8/9h8t ftp://data.pdbj.org/pub/pdb/validation_reports/h8/9h8t ftp://data.pdbj.org/pub/pdb/validation_reports/h8/9h8t | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  51939MC  9g9tC  9i4xC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 4 types, 8 molecules DdEeJjHh
| #1: Protein | Mass: 35268.703 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9R8S7 Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9R8S7#5: Protein | Mass: 7320.473 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9RB67 Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9RB67#8: Protein | Mass: 43070.770 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: Q1AL63 Chlorocebus sabaeus (green monkey) / References: UniProt: Q1AL63#10: Protein | Mass: 6623.710 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Chlorocebus sabaeus (green monkey) / Production host: Chlorocebus sabaeus (green monkey) / Production host:  Chlorocebus sabaeus (green monkey) Chlorocebus sabaeus (green monkey) |
|---|
-Cytochrome b-c1 complex subunit ... , 4 types, 9 molecules ATaGgCcFf
| #2: Protein | Mass: 29817.166 Da / Num. of mol.: 3 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) Chlorocebus sabaeus (green monkey)References: UniProt: A0A0D9QXW2, quinol-cytochrome-c reductase #3: Protein | Mass: 13540.450 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9RJ49 Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9RJ49#4: Protein | Mass: 9748.262 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9RML3 Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9RML3#6: Protein | Mass: 10803.954 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9S9I7 Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9S9I7 |
|---|
-Ubiquinol-cytochrome c reductase core protein ... , 2 types, 4 molecules KkLl
| #7: Protein | Mass: 48592.984 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9R493 Chlorocebus sabaeus (green monkey) / References: UniProt: A0A0D9R493#9: Protein | Mass: 52885.816 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  Chlorocebus sabaeus (green monkey) Chlorocebus sabaeus (green monkey) |
|---|
-Non-polymers , 10 types, 40 molecules 




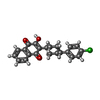

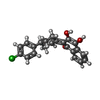











| #11: Chemical | | #12: Chemical | #13: Chemical | #14: Chemical | ChemComp-CDL / #15: Chemical | #16: Chemical | #17: Chemical | ChemComp-PEE / #18: Chemical | #19: Chemical | ChemComp-HEM / #20: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: mitochondrial respirasome from Chlorocebus sabaeus Vero cells inhibited by atovaquone Type: COMPLEX / Entity ID: #1-#10 / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Chlorocebus sabaeus (green monkey) / Strain: Vero cell line Chlorocebus sabaeus (green monkey) / Strain: Vero cell line |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 301 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1600 nm / Nominal defocus min: 600 nm / Cs: 2.7 mm |
| Image recording | Electron dose: 36 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.67 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 637526 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj























