+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9d3g | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of CCR6 bound by SQA1 and OXM1 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | MEMBRANE PROTEIN / GPCR / Antagonist / CCR6 / BRIL | ||||||
| Function / homology | : / CHOLESTEROL / Chem-EBX Function and homology information Function and homology information | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.26 Å | ||||||
 Authors Authors | Wasilko, D.J. / Wu, H. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis for CCR6 modulation by allosteric antagonists. Authors: David Jonathan Wasilko / Brian S Gerstenberger / Kathleen A Farley / Wei Li / Jennifer Alley / Mark E Schnute / Ray J Unwalla / Jorge Victorino / Kimberly K Crouse / Ru Ding / Parag V ...Authors: David Jonathan Wasilko / Brian S Gerstenberger / Kathleen A Farley / Wei Li / Jennifer Alley / Mark E Schnute / Ray J Unwalla / Jorge Victorino / Kimberly K Crouse / Ru Ding / Parag V Sahasrabudhe / Fabien Vincent / Richard K Frisbie / Alpay Dermenci / Andrew Flick / Chulho Choi / Gary Chinigo / James J Mousseau / John I Trujillo / Philippe Nuhant / Prolay Mondal / Vincent Lombardo / Daniel Lamb / Barbara J Hogan / Gurdeep Singh Minhas / Elena Segala / Christine Oswald / Ian W Windsor / Seungil Han / Mathieu Rappas / Robert M Cooke / Matthew F Calabrese / Gabriel Berstein / Atli Thorarensen / Huixian Wu /   Abstract: The CC chemokine receptor 6 (CCR6) is a potential target for chronic inflammatory diseases. Previously, we reported an active CCR6 structure in complex with its cognate chemokine CCL20, revealing the ...The CC chemokine receptor 6 (CCR6) is a potential target for chronic inflammatory diseases. Previously, we reported an active CCR6 structure in complex with its cognate chemokine CCL20, revealing the molecular basis of CCR6 activation. Here, we present two inactive CCR6 structures in ternary complexes with different allosteric antagonists, CCR6/SQA1/OXM1 and CCR6/SQA1/OXM2. The oxomorpholine analogues, OXM1 and OXM2 are highly selective CCR6 antagonists which bind to an extracellular pocket and disrupt the receptor activation network. An energetically favoured U-shaped conformation in solution that resembles the bound form is observed for the active analogues. SQA1 is a squaramide derivative with close-in analogues reported as antagonists of chemokine receptors including CCR6. SQA1 binds to an intracellular pocket which overlaps with the G protein site, stabilizing a closed pocket that is a hallmark of inactive GPCRs. Minimal communication between the two allosteric pockets is observed, in contrast to the prevalent allosteric cooperativity model of GPCRs. This work highlights the versatility of GPCR antagonism by small molecules, complementing previous knowledge of CCR6 activation, and sheds light on drug discovery targeting CCR6. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9d3g.cif.gz 9d3g.cif.gz | 204.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9d3g.ent.gz pdb9d3g.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  9d3g.json.gz 9d3g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9d3g_validation.pdf.gz 9d3g_validation.pdf.gz | 1.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9d3g_full_validation.pdf.gz 9d3g_full_validation.pdf.gz | 1.4 MB | Display | |
| Data in XML |  9d3g_validation.xml.gz 9d3g_validation.xml.gz | 49.5 KB | Display | |
| Data in CIF |  9d3g_validation.cif.gz 9d3g_validation.cif.gz | 72.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d3/9d3g https://data.pdbj.org/pub/pdb/validation_reports/d3/9d3g ftp://data.pdbj.org/pub/pdb/validation_reports/d3/9d3g ftp://data.pdbj.org/pub/pdb/validation_reports/d3/9d3g | HTTPS FTP |
-Related structure data
| Related structure data |  46534MC  9d3eC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 54623.020 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  |
|---|
-Antibody , 3 types, 3 molecules HKL
| #2: Antibody | Mass: 28832.098 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) Homo sapiens (human) |
|---|---|
| #3: Antibody | Mass: 15755.214 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host:  |
| #4: Antibody | Mass: 25343.348 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) Homo sapiens (human) |
-Non-polymers , 3 types, 3 molecules 

| #5: Chemical | ChemComp-CLR / |
|---|---|
| #6: Chemical | ChemComp-EBX / |
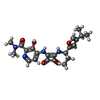
| #7: Chemical | ChemComp-A1A2A / Mass: 424.920 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C24H25ClN2O3 / Feature type: SUBJECT OF INVESTIGATION |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: CCR6-BRIL/Fab/Nb in complex with SQA1 and OXM1 / Type: COMPLEX Details: CCR6-BRIL/Fab/Nb complex co-purified with SQA1 and OXM1. Entity ID: #1-#4 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 120 kDa/nm / Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.5 Details: 50 mM HEPES pH 7.5, 150 mM NaCl, 0.003% LMNG, 0.0003% CHS, 50 uM OXM1 and 50 uM of SQA1 |
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: CCR6-BRIL/Fab/Nb complex co-purified with SQA1 and OXM1. |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 215000 X / Nominal defocus max: 2400 nm / Nominal defocus min: 800 nm / C2 aperture diameter: 100 µm |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) |
| EM imaging optics | Energyfilter name: TFS Selectris / Energyfilter slit width: 10 eV |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 5332464 | ||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.26 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 482484 / Algorithm: FOURIER SPACE / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: AB INITIO MODEL / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj









