+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8zox | ||||||
|---|---|---|---|---|---|---|---|
| Title | 3D structure of Y-50 TCR-TMM-CD1b ternary complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | IMMUNE SYSTEM | ||||||
| Function / homology |  Function and homology information Function and homology informationendogenous lipid antigen binding / exogenous lipid antigen binding / antigen processing and presentation, endogenous lipid antigen via MHC class Ib / lipopeptide binding / antigen processing and presentation, exogenous lipid antigen via MHC class Ib / early endosome lumen / Nef mediated downregulation of MHC class I complex cell surface expression / DAP12 interactions / Endosomal/Vacuolar pathway / Antigen Presentation: Folding, assembly and peptide loading of class I MHC ...endogenous lipid antigen binding / exogenous lipid antigen binding / antigen processing and presentation, endogenous lipid antigen via MHC class Ib / lipopeptide binding / antigen processing and presentation, exogenous lipid antigen via MHC class Ib / early endosome lumen / Nef mediated downregulation of MHC class I complex cell surface expression / DAP12 interactions / Endosomal/Vacuolar pathway / Antigen Presentation: Folding, assembly and peptide loading of class I MHC / negative regulation of iron ion transport / T cell mediated cytotoxicity / cellular response to iron(III) ion / negative regulation of forebrain neuron differentiation / antigen processing and presentation of exogenous protein antigen via MHC class Ib, TAP-dependent / ER to Golgi transport vesicle membrane / peptide antigen assembly with MHC class I protein complex / transferrin transport / regulation of iron ion transport / regulation of erythrocyte differentiation / negative regulation of receptor-mediated endocytosis / HFE-transferrin receptor complex / response to molecule of bacterial origin / MHC class I peptide loading complex / cellular response to iron ion / positive regulation of T cell cytokine production / antigen processing and presentation of endogenous peptide antigen via MHC class I / MHC class I protein complex / peptide antigen assembly with MHC class II protein complex / negative regulation of neurogenesis / positive regulation of receptor-mediated endocytosis / cellular response to nicotine / MHC class II protein complex / positive regulation of T cell mediated cytotoxicity / multicellular organismal-level iron ion homeostasis / specific granule lumen / peptide antigen binding / antigen processing and presentation of exogenous peptide antigen via MHC class II / phagocytic vesicle membrane / positive regulation of immune response / recycling endosome membrane / positive regulation of T cell activation / Interferon gamma signaling / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / negative regulation of epithelial cell proliferation / Modulation by Mtb of host immune system / sensory perception of smell / positive regulation of cellular senescence / tertiary granule lumen / DAP12 signaling / MHC class II protein complex binding / T cell differentiation in thymus / late endosome membrane / negative regulation of neuron projection development / ER-Phagosome pathway / protein refolding / early endosome membrane / amyloid fibril formation / protein homotetramerization / intracellular iron ion homeostasis / adaptive immune response / learning or memory / endosome membrane / immune response / endoplasmic reticulum lumen / Amyloid fiber formation / intracellular membrane-bounded organelle / Golgi membrane / external side of plasma membrane / lysosomal membrane / focal adhesion / Neutrophil degranulation / SARS-CoV-2 activates/modulates innate and adaptive immune responses / structural molecule activity / cell surface / endoplasmic reticulum / Golgi apparatus / protein homodimerization activity / extracellular space / extracellular exosome / extracellular region / identical protein binding / membrane / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.18 Å | ||||||
 Authors Authors | Asa, M. / Hirose, M. / Nagae, M. / Yamasaki, S. / Kato, T. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: J Clin Invest / Year: 2024 Journal: J Clin Invest / Year: 2024Title: A conserved human CD4+ T cell subset recognizing the mycobacterial adjuvant trehalose monomycolate. Authors: Yuki Sakai / Minori Asa / Mika Hirose / Wakana Kusuhara / Nagatoshi Fujiwara / Hiroto Tamashima / Takahiro Ikazaki / Shiori Oka / Kota Kuraba / Kentaro Tanaka / Takashi Yoshiyama / Masamichi ...Authors: Yuki Sakai / Minori Asa / Mika Hirose / Wakana Kusuhara / Nagatoshi Fujiwara / Hiroto Tamashima / Takahiro Ikazaki / Shiori Oka / Kota Kuraba / Kentaro Tanaka / Takashi Yoshiyama / Masamichi Nagae / Yoshihiko Hoshino / Daisuke Motooka / Ildiko Van Rhijn / Xiuyuan Lu / Eri Ishikawa / D Branch Moody / Takayuki Kato / Shinsuke Inuki / Go Hirai / Sho Yamasaki /    Abstract: Mycobacterium tuberculosis causes human tuberculosis (TB). As mycobacteria are protected by a thick lipid cell wall, humans have developed immune responses against diverse mycobacterial lipids. Most ...Mycobacterium tuberculosis causes human tuberculosis (TB). As mycobacteria are protected by a thick lipid cell wall, humans have developed immune responses against diverse mycobacterial lipids. Most of these immunostimulatory lipids are known as adjuvants acting through innate immune receptors, such as C-type lectin receptors. Although a few mycobacterial lipid antigens activate unconventional T cells, the antigenicity of most adjuvantic lipids is unknown. Here, we identified that trehalose monomycolate (TMM), an abundant mycobacterial adjuvant, activated human T cells bearing a unique αβ T cell receptor (αβTCR). This recognition was restricted by CD1b, a monomorphic antigen-presenting molecule conserved in primates but not mice. Single-cell TCR-RNA-Seq using newly established CD1b-TMM tetramers revealed that TMM-specific T cells were present as CD4+ effector memory T cells in the periphery of uninfected donors but expressed IFN-γ, TNF, and anti-mycobacterial effectors upon TMM stimulation. TMM-specific T cells were detected in cord blood and PBMCs of donors without bacillus Calmette-Guérin vaccination but were expanded in patients with active TB. A cryo-electron microscopy study of CD1b-TMM-TCR complexes revealed unique antigen recognition by conserved features of TCRs, positively charged CDR3α, and long CDR3β regions. These results indicate that humans have a commonly shared and preformed CD4+ T cell subset recognizing a typical mycobacterial adjuvant as an antigen. Furthermore, the dual role of TMM justifies reconsideration of the mechanism of action of adjuvants. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8zox.cif.gz 8zox.cif.gz | 222.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8zox.ent.gz pdb8zox.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8zox.json.gz 8zox.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zo/8zox https://data.pdbj.org/pub/pdb/validation_reports/zo/8zox ftp://data.pdbj.org/pub/pdb/validation_reports/zo/8zox ftp://data.pdbj.org/pub/pdb/validation_reports/zo/8zox | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  60321MC  8xubC  8zo4C C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 4 types, 4 molecules ABDE
| #1: Protein | Mass: 31005.990 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CD1B / Production host: Homo sapiens (human) / Gene: CD1B / Production host:  |
|---|---|
| #2: Protein | Mass: 11879.356 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: B2M / Production host: Homo sapiens (human) / Gene: B2M / Production host:  |
| #3: Protein | Mass: 23142.660 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Cell line (production host): HEK293 / Production host:  Homo sapiens (human) Homo sapiens (human) |
| #4: Protein | Mass: 28191.834 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Cell line (production host): HEK293 / Production host:  Homo sapiens (human) Homo sapiens (human) |
-Sugars , 2 types, 4 molecules 
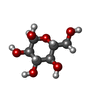

| #6: Sugar | | #7: Sugar | ChemComp-GLC / | |
|---|
-Non-polymers , 2 types, 2 molecules 
| #5: Chemical | ChemComp-6UL / |
|---|---|
| #8: Chemical | ChemComp-A1L2B / [( Mass: 658.989 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C38H74O8 / Feature type: SUBJECT OF INVESTIGATION |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Ternary complex of Y-50TCR,TMM and CD1b / Type: COMPLEX / Entity ID: #1-#4 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 8 |
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: GOLD / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil R0.6/1 |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 95 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 105000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 600 nm / Calibrated defocus min: 521 nm / Calibrated defocus max: 2036 nm / Cs: 0.06 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 78.2 K / Temperature (min): 78.2 K |
| Image recording | Average exposure time: 3.169 sec. / Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 8533 |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV / Spherical aberration corrector: CEOS corrector was used. |
| Image scans | Width: 5760 / Height: 4092 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 4075153 | ||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.18 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 114111 / Algorithm: FOURIER SPACE / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL / Target criteria: Cross-correlation coefficient | ||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 8XUB Pdb chain-ID: D / Accession code: 8XUB / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 125.22 Å2 | ||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj






