+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8qoa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of SecM-stalled Escherichia coli 70S ribosome | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | RIBOSOME / SecM / SRC / Stalling / Arrest Peptide / Arrest Motive / Stalling Motive / Stalled Ribosome Complex / SecA / Secretion Monitor / Leader Peptide / TRANSLATION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cytoplasmic translational initiation / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity ...negative regulation of cytoplasmic translational initiation / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / translation regulator activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / positive regulation of RNA splicing / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of DNA-templated transcription elongation / ribosome assembly / transcription elongation factor complex / transcription antitermination / DNA endonuclease activity / regulation of cell growth / translational initiation / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / periplasmic space / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Gersteuer, F. / Morici, M. / Wilson, D.N. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  Germany, 1items Germany, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: The SecM arrest peptide traps a pre-peptide bond formation state of the ribosome. Authors: Felix Gersteuer / Martino Morici / Sara Gabrielli / Keigo Fujiwara / Haaris A Safdari / Helge Paternoga / Lars V Bock / Shinobu Chiba / Daniel N Wilson /   Abstract: Nascent polypeptide chains can induce translational stalling to regulate gene expression. This is exemplified by the E. coli secretion monitor (SecM) arrest peptide that induces translational ...Nascent polypeptide chains can induce translational stalling to regulate gene expression. This is exemplified by the E. coli secretion monitor (SecM) arrest peptide that induces translational stalling to regulate expression of the downstream encoded SecA, an ATPase that co-operates with the SecYEG translocon to facilitate insertion of proteins into or through the cytoplasmic membrane. Here we present the structure of a ribosome stalled during translation of the full-length E. coli SecM arrest peptide at 2.0 Å resolution. The structure reveals that SecM arrests translation by stabilizing the Pro-tRNA in the A-site, but in a manner that prevents peptide bond formation with the SecM-peptidyl-tRNA in the P-site. By employing molecular dynamic simulations, we also provide insight into how a pulling force on the SecM nascent chain can relieve the SecM-mediated translation arrest. Collectively, the mechanisms determined here for SecM arrest and relief are also likely to be applicable for a variety of other arrest peptides that regulate components of the protein localization machinery identified across a wide range of bacteria lineages. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8qoa.cif.gz 8qoa.cif.gz | 3.8 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8qoa.ent.gz pdb8qoa.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8qoa.json.gz 8qoa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qo/8qoa https://data.pdbj.org/pub/pdb/validation_reports/qo/8qoa ftp://data.pdbj.org/pub/pdb/validation_reports/qo/8qoa ftp://data.pdbj.org/pub/pdb/validation_reports/qo/8qoa | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  18534MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+Large ribosomal subunit protein ... , 27 types, 27 molecules 0123cdefghijmnopqrstvwxyz4l
-RNA chain , 7 types, 7 molecules 5XZbYaA
| #5: RNA chain | Mass: 589.430 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #24: RNA chain | Mass: 2823.719 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
| #25: RNA chain | Mass: 24478.502 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #26: RNA chain | Mass: 38790.090 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #50: RNA chain | Mass: 24862.746 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #52: RNA chain | Mass: 941811.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #53: RNA chain | Mass: 499761.156 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-30S ribosomal protein ... , 5 types, 5 molecules BFGPT
| #6: Protein | Mass: 26781.670 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #10: Protein | Mass: 15727.512 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #11: Protein | Mass: 20055.156 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #18: Protein | Mass: 9207.572 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #22: Protein | Mass: 9708.464 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Small ribosomal subunit protein ... , 15 types, 15 molecules CDEHILMNOQRSUJK
| #7: Protein | Mass: 26031.316 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #8: Protein | Mass: 23514.199 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #9: Protein | Mass: 17629.398 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #12: Protein | Mass: 14146.557 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #13: Protein | Mass: 14886.270 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #14: Protein | Mass: 13814.249 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #15: Protein | Mass: 13128.467 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #16: Protein | Mass: 11606.560 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #17: Protein | Mass: 10290.816 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #19: Protein | Mass: 9724.491 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #20: Protein | Mass: 9005.472 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #21: Protein | Mass: 10455.355 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #23: Protein | Mass: 8524.039 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #54: Protein | Mass: 11755.597 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #57: Protein | Mass: 13871.959 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-50S ribosomal protein ... , 2 types, 2 molecules ku
| #35: Protein | Mass: 15008.471 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #44: Protein | Mass: 10713.465 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Protein/peptide , 1 types, 1 molecules 6
| #55: Protein/peptide | Mass: 3813.258 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 6176 molecules 


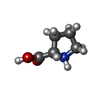





| #58: Chemical | | #59: Chemical | ChemComp-K / #60: Chemical | ChemComp-MG / #61: Chemical | ChemComp-PRO / | #62: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: SecM-stalled ribosome complex / Type: RIBOSOME / Entity ID: #1-#49, #57, #50, #56, #51-#55 / Source: MULTIPLE SOURCES |
|---|---|
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 900 nm / Nominal defocus min: 300 nm |
| Image recording | Electron dose: 40 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| EM software | Name: REFMAC / Version: 5.8.0415 / Category: model refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 300107 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 2→292.16 Å / Cor.coef. Fo:Fc: 0.91 / SU B: 3.868 / SU ML: 0.092 / ESU R: 0.104 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 71.954 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 147754 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj

































