[English] 日本語
 Yorodumi
Yorodumi- PDB-8qeh: Crystal structure of the G11 protein heterotrimer bound to FR9003... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8qeh | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the G11 protein heterotrimer bound to FR900359 inhibitor | ||||||
 Components Components | (Guanine nucleotide-binding protein ...) x 3 | ||||||
 Keywords Keywords | SIGNALING PROTEIN / G protein / FR900359 / cell signaling / GNA11 / GNB1 / GNG2 / G alpha 11 | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of melanocyte differentiation / Fatty Acids bound to GPR40 (FFAR1) regulate insulin secretion / phospholipase C-activating G protein-coupled acetylcholine receptor signaling pathway / Acetylcholine regulates insulin secretion / endothelin receptor signaling pathway / phospholipase C-activating dopamine receptor signaling pathway / developmental pigmentation / cellular response to pH / PLC beta mediated events / entrainment of circadian clock ...regulation of melanocyte differentiation / Fatty Acids bound to GPR40 (FFAR1) regulate insulin secretion / phospholipase C-activating G protein-coupled acetylcholine receptor signaling pathway / Acetylcholine regulates insulin secretion / endothelin receptor signaling pathway / phospholipase C-activating dopamine receptor signaling pathway / developmental pigmentation / cellular response to pH / PLC beta mediated events / entrainment of circadian clock / cranial skeletal system development / ligand-gated ion channel signaling pathway / action potential / phototransduction, visible light / photoreceptor outer segment / enzyme regulator activity / Turbulent (oscillatory, disturbed) flow shear stress activates signaling by PIEZO1 and integrins in endothelial cells / skeletal system development / G protein-coupled receptor binding / positive regulation of insulin secretion / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / regulation of blood pressure / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through CDC42 / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / cellular response to catecholamine stimulus / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / Inactivation, recovery and regulation of the phototransduction cascade / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / sensory perception of taste / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / heart development / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / G protein-coupled receptor signaling pathway / lysosomal membrane / GTPase activity / synapse / GTP binding / protein-containing complex binding / signal transduction / extracellular exosome / metal ion binding / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.43 Å MOLECULAR REPLACEMENT / Resolution: 1.43 Å | ||||||
 Authors Authors | Muehle, J. / Rodrigues, M.J. / Guixa-Gonzalez, R. / Deupi, X. / Schertler, G.F.X. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2025 Journal: Proc.Natl.Acad.Sci.USA / Year: 2025Title: Cyclic peptide inhibitors function as molecular glues to stabilize Gq/11 heterotrimers. Authors: Muhle, J. / Alenfelder, J. / Rodrigues, M.J. / Jurgenliemke, L. / Guixa-Gonzalez, R. / Gratz, L. / Andres, F. / Bacchin, A. / Hennig, M. / Schihada, H. / Crusemann, M. / Konig, G.M. / ...Authors: Muhle, J. / Alenfelder, J. / Rodrigues, M.J. / Jurgenliemke, L. / Guixa-Gonzalez, R. / Gratz, L. / Andres, F. / Bacchin, A. / Hennig, M. / Schihada, H. / Crusemann, M. / Konig, G.M. / Schertler, G. / Kostenis, E. / Deupi, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8qeh.cif.gz 8qeh.cif.gz | 470.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8qeh.ent.gz pdb8qeh.ent.gz | 289.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8qeh.json.gz 8qeh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8qeh_validation.pdf.gz 8qeh_validation.pdf.gz | 3.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8qeh_full_validation.pdf.gz 8qeh_full_validation.pdf.gz | 3.4 MB | Display | |
| Data in XML |  8qeh_validation.xml.gz 8qeh_validation.xml.gz | 42.8 KB | Display | |
| Data in CIF |  8qeh_validation.cif.gz 8qeh_validation.cif.gz | 61 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qe/8qeh https://data.pdbj.org/pub/pdb/validation_reports/qe/8qeh ftp://data.pdbj.org/pub/pdb/validation_reports/qe/8qeh ftp://data.pdbj.org/pub/pdb/validation_reports/qe/8qeh | HTTPS FTP |
-Related structure data
| Related structure data |  8qegC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules ABG
| #1: Protein | Mass: 41139.887 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNA11, GA11 / Production host: Homo sapiens (human) / Gene: GNA11, GA11 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P29992 Trichoplusia ni (cabbage looper) / References: UniProt: P29992 |
|---|---|
| #2: Protein | Mass: 37671.102 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNB1 / Production host: Homo sapiens (human) / Gene: GNB1 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P62873 Trichoplusia ni (cabbage looper) / References: UniProt: P62873 |
| #3: Protein | Mass: 7845.078 Da / Num. of mol.: 1 / Mutation: C68S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNG2 / Production host: Homo sapiens (human) / Gene: GNG2 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P59768 Trichoplusia ni (cabbage looper) / References: UniProt: P59768 |
-Non-polymers , 14 types, 782 molecules 



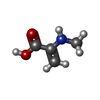
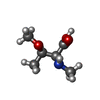















| #4: Chemical | ChemComp-EDO / #5: Chemical | ChemComp-GDP / | #6: Chemical | ChemComp-ZN / | #7: Chemical | ChemComp-CL / | #8: Chemical | ChemComp-DAM / | #9: Chemical | ChemComp-HF2 / ( | #10: Chemical | ChemComp-UDL / ( | Mass: 189.209 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C8H15NO4 / Feature type: SUBJECT OF INVESTIGATION #11: Chemical | ChemComp-OTH / | #12: Chemical | #13: Chemical | ChemComp-MAA / | #14: Chemical | ChemComp-ALA / | #15: Chemical | ChemComp-PPI / | #16: Chemical | #17: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.52 Å3/Da / Density % sol: 51.16 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 4.5 Details: 0.09 M Na Acetate pH 4.5, 2.7 % PEG Smears Medium, 6.3 % MPD, 0.5% n-octyl-beta-D-Glucoside and 10 mM Zinc sulfate heptahydrate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06DA / Wavelength: 1 Å / Beamline: X06DA / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 2M-F / Detector: PIXEL / Date: Jan 28, 2022 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.43→126.8 Å / Num. obs: 163494 / % possible obs: 100 % / Redundancy: 44 % / CC1/2: 1 / Net I/σ(I): 16.5 |
| Reflection shell | Resolution: 1.43→1.45 Å / Redundancy: 46.7 % / Mean I/σ(I) obs: 0.7 / Num. unique obs: 8016 / CC1/2: 0.309 / % possible all: 99.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.43→63.113 Å / Cor.coef. Fo:Fc: 0.979 / Cor.coef. Fo:Fc free: 0.972 / WRfactor Rfree: 0.16 / WRfactor Rwork: 0.124 / SU B: 3.456 / SU ML: 0.054 / Average fsc free: 0.9661 / Average fsc work: 0.9788 / Cross valid method: THROUGHOUT / ESU R: 0.056 / ESU R Free: 0.056 MOLECULAR REPLACEMENT / Resolution: 1.43→63.113 Å / Cor.coef. Fo:Fc: 0.979 / Cor.coef. Fo:Fc free: 0.972 / WRfactor Rfree: 0.16 / WRfactor Rwork: 0.124 / SU B: 3.456 / SU ML: 0.054 / Average fsc free: 0.9661 / Average fsc work: 0.9788 / Cross valid method: THROUGHOUT / ESU R: 0.056 / ESU R Free: 0.056 Details: Hydrogens have been added in their riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.3 Å / Solvent model: MASK BULK SOLVENT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.784 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.43→63.113 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller


 PDBj
PDBj































