[English] 日本語
 Yorodumi
Yorodumi- PDB-8pu0: Cryo-EM structure of human Elp123 in complex with tRNA, desulpho-... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8pu0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human Elp123 in complex with tRNA, desulpho-CoA, 5'-deoxyadenosine and methionine | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSLATION / Elongator / tRNA modification / acetyl-CoA hydrolysis | ||||||
| Function / homology |  Function and homology information Function and homology informationphosphorylase kinase regulator activity / tRNA uridine(34) acetyltransferase activity / tRNA carboxymethyluridine synthase / elongator holoenzyme complex / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / regulation of receptor signaling pathway via JAK-STAT / acetyltransferase activity / central nervous system development / transcription elongation factor complex ...phosphorylase kinase regulator activity / tRNA uridine(34) acetyltransferase activity / tRNA carboxymethyluridine synthase / elongator holoenzyme complex / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / regulation of receptor signaling pathway via JAK-STAT / acetyltransferase activity / central nervous system development / transcription elongation factor complex / transcription elongation by RNA polymerase II / neuron migration / regulation of translation / HATs acetylate histones / 4 iron, 4 sulfur cluster binding / tRNA binding / positive regulation of cell migration / regulation of transcription by RNA polymerase II / protein kinase binding / nucleolus / nucleoplasm / metal ion binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.25 Å | ||||||
 Authors Authors | Abbassi, N. / Jaciuk, M. / Lin, T.-Y. / Glatt, S. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Cryo-EM structures of the human Elongator complex at work. Authors: Nour-El-Hana Abbassi / Marcin Jaciuk / David Scherf / Pauline Böhnert / Alexander Rau / Alexander Hammermeister / Michał Rawski / Paulina Indyka / Grzegorz Wazny / Andrzej Chramiec- ...Authors: Nour-El-Hana Abbassi / Marcin Jaciuk / David Scherf / Pauline Böhnert / Alexander Rau / Alexander Hammermeister / Michał Rawski / Paulina Indyka / Grzegorz Wazny / Andrzej Chramiec-Głąbik / Dominika Dobosz / Bozena Skupien-Rabian / Urszula Jankowska / Juri Rappsilber / Raffael Schaffrath / Ting-Yu Lin / Sebastian Glatt /    Abstract: tRNA modifications affect ribosomal elongation speed and co-translational folding dynamics. The Elongator complex is responsible for introducing 5-carboxymethyl at wobble uridine bases (cmU) in ...tRNA modifications affect ribosomal elongation speed and co-translational folding dynamics. The Elongator complex is responsible for introducing 5-carboxymethyl at wobble uridine bases (cmU) in eukaryotic tRNAs. However, the structure and function of human Elongator remain poorly understood. In this study, we present a series of cryo-EM structures of human ELP123 in complex with tRNA and cofactors at four different stages of the reaction. The structures at resolutions of up to 2.9 Å together with complementary functional analyses reveal the molecular mechanism of the modification reaction. Our results show that tRNA binding exposes a universally conserved uridine at position 33 (U), which triggers acetyl-CoA hydrolysis. We identify a series of conserved residues that are crucial for the radical-based acetylation of U and profile the molecular effects of patient-derived mutations. Together, we provide the high-resolution view of human Elongator and reveal its detailed mechanism of action. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8pu0.cif.gz 8pu0.cif.gz | 534.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8pu0.ent.gz pdb8pu0.ent.gz | 411.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8pu0.json.gz 8pu0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8pu0_validation.pdf.gz 8pu0_validation.pdf.gz | 1.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8pu0_full_validation.pdf.gz 8pu0_full_validation.pdf.gz | 1.5 MB | Display | |
| Data in XML |  8pu0_validation.xml.gz 8pu0_validation.xml.gz | 100.1 KB | Display | |
| Data in CIF |  8pu0_validation.cif.gz 8pu0_validation.cif.gz | 150.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pu/8pu0 https://data.pdbj.org/pub/pdb/validation_reports/pu/8pu0 ftp://data.pdbj.org/pub/pdb/validation_reports/pu/8pu0 ftp://data.pdbj.org/pub/pdb/validation_reports/pu/8pu0 | HTTPS FTP |
-Related structure data
| Related structure data |  17927MC  8ptxC  8ptyC  8ptzC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Elongator complex protein ... , 3 types, 4 molecules AEBC
| #1: Protein | Mass: 150427.484 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ELP1, IKAP, IKBKAP / Production host: Homo sapiens (human) / Gene: ELP1, IKAP, IKBKAP / Production host:  #2: Protein | | Mass: 92597.766 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ELP2, STATIP1 / Production host: Homo sapiens (human) / Gene: ELP2, STATIP1 / Production host:  #3: Protein | | Mass: 65740.539 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ELP3 / Production host: Homo sapiens (human) / Gene: ELP3 / Production host:  References: UniProt: Q9H9T3, Transferases; Acyltransferases; Transferring groups other than aminoacyl groups |
|---|
-RNA chain , 1 types, 1 molecules X
| #4: RNA chain | Mass: 24047.236 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)  Homo sapiens (human) Homo sapiens (human) |
|---|
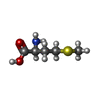
-Non-polymers , 4 types, 4 molecules 





| #5: Chemical | ChemComp-DCA / |
|---|---|
| #6: Chemical | ChemComp-SF4 / |
| #7: Chemical | ChemComp-5AD / |
| #8: Chemical | ChemComp-MET / |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Human Elp123 in complex with glutamine tRNA, desulpho-CoA, 5'-deoxyadenosine and methionine Type: COMPLEX / Entity ID: #1-#4 / Source: RECOMBINANT | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.635 MDa / Experimental value: NO | |||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||
| Source (recombinant) | Organism:  | |||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | |||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||
| Specimen | Conc.: 0.6 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||||||||||||
| Specimen support | Details: 8 mA / Grid material: COPPER / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil R2/1 | |||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K / Details: 15 s wait time, blot force 5, 5 s blot time |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 105000 X / Nominal defocus max: 2100 nm / Nominal defocus min: 900 nm / Cs: 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 41.22 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of real images: 20720 |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV |
| Image scans | Width: 5760 / Height: 4092 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image processing | Details: 20 eV slit, fully tuned before the experiment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.25 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 115026 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Details: Elp123-tRNA-ACO structure from the same study / Source name: Other / Type: in silico model | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





 PDBj
PDBj


































