+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8oyg | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of ASBTNM in complex with pantoate | ||||||
 Components Components | Transporter | ||||||
 Keywords Keywords | TRANSPORT PROTEIN / Secondary Membrane Transporter | ||||||
| Function / homology | Bile acid:sodium symporter/arsenical resistance protein Acr3 / Bile acid:sodium symporter / Sodium Bile acid symporter family / Sodium/solute symporter superfamily / membrane / : / (2R)-2,3-dihydroxypropyl (9Z)-octadec-9-enoate / PANTOATE / Transporter Function and homology information Function and homology information | ||||||
| Biological species |  Neisseria meningitidis MC58 (bacteria) Neisseria meningitidis MC58 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Becker, P. / Cameron, A.D. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Biorxiv / Year: 2023 Journal: Biorxiv / Year: 2023Title: Mechanism of substrate binding and transport in BASS transporters. Authors: Becker, P. / Naughton, F.B. / Brotherton, D.H. / Pacheco-Gomez, R. / Beckstein, O. / Cameron, A.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8oyg.cif.gz 8oyg.cif.gz | 220.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8oyg.ent.gz pdb8oyg.ent.gz | 146.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8oyg.json.gz 8oyg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oy/8oyg https://data.pdbj.org/pub/pdb/validation_reports/oy/8oyg ftp://data.pdbj.org/pub/pdb/validation_reports/oy/8oyg ftp://data.pdbj.org/pub/pdb/validation_reports/oy/8oyg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8oyfC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 33836.625 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Neisseria meningitidis MC58 (bacteria) / Gene: NMB0705 / Production host: Neisseria meningitidis MC58 (bacteria) / Gene: NMB0705 / Production host:  |
|---|
-Non-polymers , 5 types, 31 molecules 
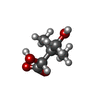







| #2: Chemical | | #3: Chemical | ChemComp-PAF / | #4: Chemical | ChemComp-OLC / ( | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.46 Å3/Da / Density % sol: 49.91 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: lipidic cubic phase / pH: 7.5 Details: 0.2 M Magnesium chloride hexahydrate, 0.005 M Cadmium chloride hemi-(pentahydrate), 0.1 M Tris (pH 7.5), and 14% v/v PEG 500 MME |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.9999 Å / Beamline: I24 / Wavelength: 0.9999 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Dec 12, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9999 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→44 Å / Num. obs: 14273 / % possible obs: 97.74 % / Redundancy: 3.9 % / Biso Wilson estimate: 35.55 Å2 / CC1/2: 0.988 / CC star: 0.997 / Rmerge(I) obs: 0.1181 / Rpim(I) all: 0.06752 / Rrim(I) all: 0.1368 / Net I/σ(I): 5.74 |
| Reflection shell | Resolution: 2.3→2.382 Å / Redundancy: 2.5 % / Rmerge(I) obs: 0.4632 / Num. unique obs: 1201 / CC1/2: 0.804 / CC star: 0.944 / Rpim(I) all: 0.3397 / Rrim(I) all: 0.5779 / % possible all: 82.77 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.3→44 Å / SU ML: 0.265 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 33.5519 MOLECULAR REPLACEMENT / Resolution: 2.3→44 Å / SU ML: 0.265 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 33.5519 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 52.19 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→44 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 9.03662140082 Å / Origin y: 11.2184429355 Å / Origin z: 16.5014518344 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller



 PDBj
PDBj








