[English] 日本語
 Yorodumi
Yorodumi- PDB-8oqu: Structure of Mycobacterium tuberculosis beta-oxidation trifunctio... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8oqu | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Mycobacterium tuberculosis beta-oxidation trifunctional enzyme in complex with Fragment-M-92 | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / fatty acid beta oxidation complex / mycobacterium tuberculosis / TFE / fragment screening / substrate channeling | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationlong-chain (3S)-3-hydroxyacyl-CoA dehydrogenase (NAD+) activity / acetyl-CoA C-acetyltransferase activity / enoyl-CoA hydratase activity / fatty acid beta-oxidation / NAD+ binding / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / peptidoglycan-based cell wall / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.89 Å MOLECULAR REPLACEMENT / Resolution: 2.89 Å | ||||||||||||
 Authors Authors | Dalwani, S. / Wierenga, R.K. / Venkatesan, R. | ||||||||||||
| Funding support |  Finland, 3items Finland, 3items
| ||||||||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2024 Journal: Acta Crystallogr D Struct Biol / Year: 2024Title: Crystallographic fragment-binding studies of the Mycobacterium tuberculosis trifunctional enzyme suggest binding pockets for the tails of the acyl-CoA substrates at its active sites and a ...Title: Crystallographic fragment-binding studies of the Mycobacterium tuberculosis trifunctional enzyme suggest binding pockets for the tails of the acyl-CoA substrates at its active sites and a potential substrate-channeling path between them. Authors: Dalwani, S. / Metz, A. / Huschmann, F.U. / Weiss, M.S. / Wierenga, R.K. / Venkatesan, R. #1:  Journal: Biorxiv / Year: 2024 Journal: Biorxiv / Year: 2024Title: Crystallographic fragment binding studies of the Mycobacterium tuberculosis trifunctional enzyme suggest binding pockets for the tails of the acyl-CoA substrates at its active sites and a ...Title: Crystallographic fragment binding studies of the Mycobacterium tuberculosis trifunctional enzyme suggest binding pockets for the tails of the acyl-CoA substrates at its active sites and a potential substrate channeling path between them Authors: Dalwani, S. / Metz, A. / Huschmann, F.U. / Weiss, M.S. / Wierenga, R.K. / Venkatesan, R. #2:  Journal: J Struct Biol / Year: 2021 Journal: J Struct Biol / Year: 2021Title: Substrate specificity and conformational flexibility properties of the Mycobacterium tuberculosis beta-oxidation trifunctional enzyme. Authors: Dalwani, S. #3:  Journal: ACS Chem Biol / Year: 2013 Journal: ACS Chem Biol / Year: 2013Title: Structure of mycobacterial beta-oxidation trifunctional enzyme reveals its altered assembly and putative substrate channeling pathway. Authors: Venkatesan, R. / Wierenga, R.K. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8oqu.cif.gz 8oqu.cif.gz | 1007.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8oqu.ent.gz pdb8oqu.ent.gz | 696.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8oqu.json.gz 8oqu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oq/8oqu https://data.pdbj.org/pub/pdb/validation_reports/oq/8oqu ftp://data.pdbj.org/pub/pdb/validation_reports/oq/8oqu ftp://data.pdbj.org/pub/pdb/validation_reports/oq/8oqu | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8opuC  8opvC  8opwC  8opxC  8opyC  8oqlC  8oqmC  8oqnC  8oqoC  8oqpC  8oqqC  8oqrC  8oqsC  8oqtC  8oqvC  8pf8C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 2 types, 4 molecules ABCD
| #1: Protein | Mass: 78005.805 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria)Gene: fadB, Rv0860 / Production host:  References: UniProt: O53872, 3-hydroxyacyl-CoA dehydrogenase #2: Protein | Mass: 42460.355 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria)Gene: fadA, Rv0859 / Production host:  References: UniProt: O53871, Transferases; Acyltransferases; Transferring groups other than aminoacyl groups |
|---|
-Non-polymers , 4 types, 93 molecules 

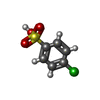




| #3: Chemical | ChemComp-SO4 / #4: Chemical | ChemComp-GOL / #5: Chemical | ChemComp-VXN / #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.94 Å3/Da / Density % sol: 68.81 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 8.5 / Details: 2 M Ammonium Sulfate, 0.1M Tris pH 8.5 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  MAX IV MAX IV  / Beamline: BioMAX / Wavelength: 0.976254 Å / Beamline: BioMAX / Wavelength: 0.976254 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Nov 1, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976254 Å / Relative weight: 1 |
| Reflection | Resolution: 2.89→116.86 Å / Num. obs: 64333 / % possible obs: 93.2 % / Redundancy: 6.9 % / Biso Wilson estimate: 77.64 Å2 / CC1/2: 0.992 / Net I/σ(I): 8.5 |
| Reflection shell | Resolution: 2.89→3.17 Å / Num. unique obs: 5362 / CC1/2: 0.498 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.89→116.86 Å / SU ML: 0.3137 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 25.2837 MOLECULAR REPLACEMENT / Resolution: 2.89→116.86 Å / SU ML: 0.3137 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 25.2837 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 75.3 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.89→116.86 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -43.7986172894 Å / Origin y: 34.2542621323 Å / Origin z: -1.74521507425 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller


 PDBj
PDBj







