+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8omx | ||||||
|---|---|---|---|---|---|---|---|
| Title | NI,FE-CODH -600mV state : 1 min Dioxygen Exposure | ||||||
 Components Components | Carbon monoxide dehydrogenase 2 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / CLUSTER C / 4FE-4S / CYTOPLASM / IRON / IRON-SULFUR / MEMBRANE / METAL-BINDING / NICKEL | ||||||
| Function / homology |  Function and homology information Function and homology informationanaerobic carbon monoxide dehydrogenase / hydroxylamine reductase activity / anaerobic carbon-monoxide dehydrogenase activity / nickel cation binding / generation of precursor metabolites and energy / peroxidase activity / response to hydrogen peroxide / 4 iron, 4 sulfur cluster binding / plasma membrane / cytoplasm Similarity search - Function | ||||||
| Biological species |   Carboxydothermus hydrogenoformans Z-2901 (bacteria) Carboxydothermus hydrogenoformans Z-2901 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Basak, Y. / Jeoung, J.-H. / Dobbek, H. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Angew.Chem.Int.Ed.Engl. / Year: 2023 Journal: Angew.Chem.Int.Ed.Engl. / Year: 2023Title: Stepwise O 2 -Induced Rearrangement and Disassembly of the [NiFe 4 (OH)( mu 3 -S) 4 ] Active Site Cluster of CO Dehydrogenase. Authors: Basak, Y. / Jeoung, J.H. / Domnik, L. / Dobbek, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8omx.cif.gz 8omx.cif.gz | 368.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8omx.ent.gz pdb8omx.ent.gz | 302.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8omx.json.gz 8omx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/om/8omx https://data.pdbj.org/pub/pdb/validation_reports/om/8omx ftp://data.pdbj.org/pub/pdb/validation_reports/om/8omx ftp://data.pdbj.org/pub/pdb/validation_reports/om/8omx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8omyC  8on0C  8on1C  8on2C  8on3C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules X
| #1: Protein | Mass: 66992.477 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Carboxydothermus hydrogenoformans Z-2901 (bacteria) Carboxydothermus hydrogenoformans Z-2901 (bacteria)Strain: Z-2901 / DSM 6008 / Gene: cooS2, cooSII, CHY_0085 / Plasmid: pET28A / Production host:  References: UniProt: Q9F8A8, anaerobic carbon monoxide dehydrogenase |
|---|
-Non-polymers , 6 types, 576 molecules 

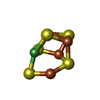




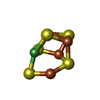



| #2: Chemical | ChemComp-SF4 / |
|---|---|
| #3: Chemical | ChemComp-FES / |
| #4: Chemical | ChemComp-NFS / |
| #5: Chemical | ChemComp-FE2 / |
| #6: Chemical | ChemComp-H2S / |
| #7: Water | ChemComp-HOH / |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.11 Å3/Da / Density % sol: 41.7 % |
|---|---|
| Crystal grow | Temperature: 291.15 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 0.1M Bis-Tris pH 6.5, PEG 3350 10-18%, Ammonium sulphate 0.18M |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.1 / Wavelength: 0.91841 Å / Beamline: 14.1 / Wavelength: 0.91841 Å |
| Detector | Type: DECTRIS PILATUS3 S 6M / Detector: PIXEL / Date: Nov 10, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.91841 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→40.85 Å / Num. obs: 83154 / % possible obs: 94 % / Redundancy: 3.38 % / CC1/2: 0.999 / Net I/σ(I): 0.115 |
| Reflection shell | Resolution: 1.5→1.56 Å / Num. unique obs: 7758 / CC1/2: 0.658 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.5→40.82 Å / SU ML: 0.15 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 20.54 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 1.5→40.82 Å / SU ML: 0.15 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 20.54 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→40.82 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj
















