| 登録情報 | データベース: PDB / ID: 8hlj
|
|---|
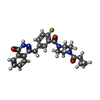
| タイトル | Mutated human ADP-ribosyltransferase 2 (PARP2) catalytic domain bound to Olaparib (AZD2281) |
|---|
 要素 要素 | Poly [ADP-ribose] polymerase 2 |
|---|
 キーワード キーワード | TRANSFERASE / DNA ADP-ribosyltransferase 2 / PARP2 / Olaparib / AZD2281 |
|---|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報
response to oxygen-glucose deprivation / hippocampal neuron apoptotic process / poly-ADP-D-ribose binding / positive regulation of cell growth involved in cardiac muscle cell development / NAD+-protein-serine ADP-ribosyltransferase activity / NAD DNA ADP-ribosyltransferase activity / DNA ADP-ribosylation / poly-ADP-D-ribose modification-dependent protein binding / HDR through MMEJ (alt-NHEJ) / NAD+ ADP-ribosyltransferase ...response to oxygen-glucose deprivation / hippocampal neuron apoptotic process / poly-ADP-D-ribose binding / positive regulation of cell growth involved in cardiac muscle cell development / NAD+-protein-serine ADP-ribosyltransferase activity / NAD DNA ADP-ribosyltransferase activity / DNA ADP-ribosylation / poly-ADP-D-ribose modification-dependent protein binding / HDR through MMEJ (alt-NHEJ) / NAD+ ADP-ribosyltransferase / protein auto-ADP-ribosylation / NAD+-protein-aspartate ADP-ribosyltransferase activity / protein poly-ADP-ribosylation / NAD+-protein-glutamate ADP-ribosyltransferase activity / DNA repair-dependent chromatin remodeling / NAD+-protein mono-ADP-ribosyltransferase activity / decidualization / site of DNA damage / 転移酵素; グリコシル基を移すもの; 五炭糖残基を移すもの / NAD+ poly-ADP-ribosyltransferase activity / POLB-Dependent Long Patch Base Excision Repair / nucleosome binding / extrinsic apoptotic signaling pathway / nucleotidyltransferase activity / base-excision repair / DNA Damage Recognition in GG-NER / Dual Incision in GG-NER / Formation of Incision Complex in GG-NER / double-strand break repair / damaged DNA binding / DNA repair / DNA damage response / chromatin binding / nucleolus / nucleoplasm / nucleus / cytosol類似検索 - 分子機能 : / Poly(ADP-ribose) polymerase, regulatory domain / WGR domain / WGR domain superfamily / WGR domain / WGR domain profile. / Proposed nucleic acid binding domain / Poly(ADP-ribose) polymerase, regulatory domain superfamily / Poly(ADP-ribose) polymerase, regulatory domain / PARP alpha-helical domain profile. ...: / Poly(ADP-ribose) polymerase, regulatory domain / WGR domain / WGR domain superfamily / WGR domain / WGR domain profile. / Proposed nucleic acid binding domain / Poly(ADP-ribose) polymerase, regulatory domain superfamily / Poly(ADP-ribose) polymerase, regulatory domain / PARP alpha-helical domain profile. / Poly(ADP-ribose) polymerase catalytic domain / Poly(ADP-ribose) polymerase, catalytic domain / PARP catalytic domain profile.類似検索 - ドメイン・相同性 |
|---|
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) |
|---|
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 2.24 Å 分子置換 / 解像度: 2.24 Å |
|---|
 データ登録者 データ登録者 | Wang, X.Y. / Zhou, J. / Xu, B.L. |
|---|
| 資金援助 |  中国, 3件 中国, 3件 | 組織 | 認可番号 | 国 |
|---|
| National Natural Science Foundation of China (NSFC) | 82003657 |  中国 中国 | | National Natural Science Foundation of China (NSFC) | 81673300 |  中国 中国 | | National Natural Science Foundation of China (NSFC) | 82173693 |  中国 中国 |
|
|---|
 引用 引用 |  ジャーナル: Bioorg.Chem. / 年: 2025 ジャーナル: Bioorg.Chem. / 年: 2025
タイトル: Engaging an engineered PARP-2 catalytic domain mutant to solve the complex structures harboring approved drugs for structure analyses.
著者: Wang, X. / Zhou, J. / Xu, B. |
|---|
| 履歴 | | 登録 | 2022年11月30日 | 登録サイト: PDBJ / 処理サイト: PDBC |
|---|
| 改定 1.0 | 2024年5月1日 | Provider: repository / タイプ: Initial release |
|---|
| 改定 1.1 | 2025年5月14日 | Group: Database references / Structure summary
カテゴリ: citation / citation_author / pdbx_entry_details
Item: _citation.country / _citation.journal_abbrev ..._citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _citation_author.name / _pdbx_entry_details.has_protein_modification |
|---|
|
|---|
 データを開く
データを開く 基本情報
基本情報 要素
要素 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) X線回折 /
X線回折 /  シンクロトロン /
シンクロトロン /  分子置換 / 解像度: 2.24 Å
分子置換 / 解像度: 2.24 Å  データ登録者
データ登録者 中国, 3件
中国, 3件  引用
引用 ジャーナル: Bioorg.Chem. / 年: 2025
ジャーナル: Bioorg.Chem. / 年: 2025 構造の表示
構造の表示 Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク ダウンロード
ダウンロード 8hlj.cif.gz
8hlj.cif.gz PDBx/mmCIF形式
PDBx/mmCIF形式 pdb8hlj.ent.gz
pdb8hlj.ent.gz PDB形式
PDB形式 8hlj.json.gz
8hlj.json.gz PDBx/mmJSON形式
PDBx/mmJSON形式 その他のダウンロード
その他のダウンロード 8hlj_validation.pdf.gz
8hlj_validation.pdf.gz wwPDB検証レポート
wwPDB検証レポート 8hlj_full_validation.pdf.gz
8hlj_full_validation.pdf.gz 8hlj_validation.xml.gz
8hlj_validation.xml.gz 8hlj_validation.cif.gz
8hlj_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/hl/8hlj
https://data.pdbj.org/pub/pdb/validation_reports/hl/8hlj ftp://data.pdbj.org/pub/pdb/validation_reports/hl/8hlj
ftp://data.pdbj.org/pub/pdb/validation_reports/hl/8hlj リンク
リンク 集合体
集合体


 要素
要素 Homo sapiens (ヒト) / 遺伝子: PARP2, ADPRT2, ADPRTL2 / 発現宿主:
Homo sapiens (ヒト) / 遺伝子: PARP2, ADPRT2, ADPRTL2 / 発現宿主: 
 X線回折 / 使用した結晶の数: 1
X線回折 / 使用した結晶の数: 1  試料調製
試料調製 シンクロトロン / サイト:
シンクロトロン / サイト:  SSRF
SSRF  / ビームライン: BL02U1 / 波長: 0.979183 Å
/ ビームライン: BL02U1 / 波長: 0.979183 Å 解析
解析 分子置換
分子置換 ムービー
ムービー コントローラー
コントローラー








 PDBj
PDBj