[English] 日本語
 Yorodumi
Yorodumi- PDB-8gkc: Atomic model of the core modifying region of human fatty acid syn... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8gkc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Atomic model of the core modifying region of human fatty acid synthase in complex with TVB-2640 - C2 refinement | |||||||||
 Components Components | Fatty acid synthase | |||||||||
 Keywords Keywords | TRANSFERASE/INHIBITOR / Fatty acid synthase / TRANSFERASE / TRANSFERASE-INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationfatty-acid synthase system / ether lipid biosynthetic process / Vitamin B5 (pantothenate) metabolism / neutrophil differentiation / fatty-acyl-CoA biosynthetic process / enoyl-[acyl-carrier-protein] reductase (NADPH, Re-specific) / establishment of endothelial intestinal barrier / glycogen granule / [acyl-carrier-protein] S-acetyltransferase / [acyl-carrier-protein] S-acetyltransferase activity ...fatty-acid synthase system / ether lipid biosynthetic process / Vitamin B5 (pantothenate) metabolism / neutrophil differentiation / fatty-acyl-CoA biosynthetic process / enoyl-[acyl-carrier-protein] reductase (NADPH, Re-specific) / establishment of endothelial intestinal barrier / glycogen granule / [acyl-carrier-protein] S-acetyltransferase / [acyl-carrier-protein] S-acetyltransferase activity / Fatty acyl-CoA biosynthesis / host-mediated perturbation of viral process / ChREBP activates metabolic gene expression / enoyl-[acyl-carrier-protein] reductase (NADPH) activity / [acyl-carrier-protein] S-malonyltransferase / [acyl-carrier-protein] S-malonyltransferase activity / 3-hydroxyacyl-[acyl-carrier-protein] dehydratase / (3R)-hydroxyacyl-[acyl-carrier-protein] dehydratase activity / acetyl-CoA metabolic process / beta-ketoacyl-[acyl-carrier-protein] synthase I / NR1H2 & NR1H3 regulate gene expression linked to lipogenesis / 3-oxoacyl-[acyl-carrier-protein] reductase (NADPH) activity / 3-oxoacyl-[acyl-carrier-protein] reductase / mammary gland development / oleoyl-[acyl-carrier-protein] hydrolase / fatty acyl-[ACP] hydrolase activity / fatty acid synthase activity / monocyte differentiation / phosphopantetheine binding / 3-oxoacyl-[acyl-carrier-protein] synthase activity / response to nutrient / cellular response to interleukin-4 / Activation of gene expression by SREBF (SREBP) / fatty acid metabolic process / fatty acid biosynthetic process / osteoblast differentiation / melanosome / cadherin binding / inflammatory response / Golgi apparatus / RNA binding / extracellular exosome / identical protein binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.45 Å | |||||||||
 Authors Authors | Hasan, S.M.N. / Keszei, A. / Mazhab-Jafari, M.T. | |||||||||
| Funding support |  Canada, 2items Canada, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Atomic model for core modifying region of human fatty acid synthase in complex with Denifanstat. Authors: S M Naimul Hasan / Jennifer W Lou / Alexander F A Keszei / David L Dai / Mohammad T Mazhab-Jafari /  Abstract: Fatty acid synthase (FASN) catalyzes the de novo synthesis of palmitate, a 16-carbon chain fatty acid that is the primary precursor of lipid metabolism and an important intracellular signaling ...Fatty acid synthase (FASN) catalyzes the de novo synthesis of palmitate, a 16-carbon chain fatty acid that is the primary precursor of lipid metabolism and an important intracellular signaling molecule. FASN is an attractive drug target in diabetes, cancer, fatty liver diseases, and viral infections. Here, we develop an engineered full-length human FASN (hFASN) that enables isolation of the condensing and modifying regions of the protein post-translation. The engineered protein enables electron cryo-microscopy (cryoEM) structure determination of the core modifying region of hFASN to 2.7 Å resolution. Examination of the dehydratase dimer within this region reveals that unlike its close homolog, porcine FASN, the catalytic cavity is close-ended and is accessible only through one opening in the vicinity of the active site. The core modifying region exhibits two major global conformational variabilities that describe long-range bending and twisting motions of the complex in solution. Finally, we solved the structure of this region bound to an anti-cancer drug, Denifanstat (i.e., TVB-2640), demonstrating the utility of our approach as a platform for structure guided design of future hFASN small molecule inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8gkc.cif.gz 8gkc.cif.gz | 408.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8gkc.ent.gz pdb8gkc.ent.gz | 303.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8gkc.json.gz 8gkc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gk/8gkc https://data.pdbj.org/pub/pdb/validation_reports/gk/8gkc ftp://data.pdbj.org/pub/pdb/validation_reports/gk/8gkc ftp://data.pdbj.org/pub/pdb/validation_reports/gk/8gkc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  40182MC  8eyiC  8eykC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
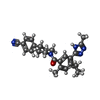
| #1: Protein | Mass: 182749.016 Da / Num. of mol.: 2 / Fragment: residues 855-2511 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: FASN, FAS / Production host: Homo sapiens (human) / Gene: FASN, FAS / Production host:  Homo sapiens (human) Homo sapiens (human)References: UniProt: P49327, fatty-acid synthase system, [acyl-carrier-protein] S-acetyltransferase, [acyl-carrier-protein] S-malonyltransferase, beta-ketoacyl-[acyl-carrier-protein] synthase I, 3- ...References: UniProt: P49327, fatty-acid synthase system, [acyl-carrier-protein] S-acetyltransferase, [acyl-carrier-protein] S-malonyltransferase, beta-ketoacyl-[acyl-carrier-protein] synthase I, 3-oxoacyl-[acyl-carrier-protein] reductase, 3-hydroxyacyl-[acyl-carrier-protein] dehydratase, enoyl-[acyl-carrier-protein] reductase (NADPH, Re-specific), oleoyl-[acyl-carrier-protein] hydrolase #2: Chemical | #3: Chemical | ChemComp-NDP / Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Fatty acid synthase / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.34 MDa / Experimental value: YES |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) / Cell: HEK293 / Plasmid: pcDNA3.1 Homo sapiens (human) / Cell: HEK293 / Plasmid: pcDNA3.1 |
| Buffer solution | pH: 7.4 |
| Specimen | Conc.: 0.8 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: COPPER/RHODIUM / Grid mesh size: 400 divisions/in. / Grid type: Homemade |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2500 nm / Nominal defocus min: 600 nm / Cs: 2.7 mm |
| Image recording | Electron dose: 50.76 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 4801 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20.1_4487: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.45 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 311983 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj