Entry Database : PDB / ID : 8g6aTitle Wildtype PTP1b in complex with DES6016 Tyrosine-protein phosphatase non-receptor type 1 Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 1.62 Å Authors Greisman, J.B. / Willmore, L. / Yeh, C.Y. / Giordanetto, F. / Shahamadtar, S. / Nisonoff, H. / Maragakis, P. / Shaw, D.E. Funding support 1items Organization Grant number Country Not funded
Journal : J.Chem.Inf.Model. / Year : 2023Title : Discovery and Validation of the Binding Poses of Allosteric Fragment Hits to Protein Tyrosine Phosphatase 1b: From Molecular Dynamics Simulations to X-ray Crystallography.Authors : Greisman, J.B. / Willmore, L. / Yeh, C.Y. / Giordanetto, F. / Shahamadtar, S. / Nisonoff, H. / Maragakis, P. / Shaw, D.E. History Deposition Feb 14, 2023 Deposition site / Processing site Revision 1.0 Apr 26, 2023 Provider / Type Revision 1.1 May 3, 2023 Group / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _citation_author.identifier_ORCID Revision 1.2 May 17, 2023 Group / Category Item / _citation.page_first / _citation.page_lastRevision 1.3 May 22, 2024 Group / Category / chem_comp_bond
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.62 Å
MOLECULAR REPLACEMENT / Resolution: 1.62 Å  Authors
Authors Citation
Citation Journal: J.Chem.Inf.Model. / Year: 2023
Journal: J.Chem.Inf.Model. / Year: 2023 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 8g6a.cif.gz
8g6a.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb8g6a.ent.gz
pdb8g6a.ent.gz PDB format
PDB format 8g6a.json.gz
8g6a.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/g6/8g6a
https://data.pdbj.org/pub/pdb/validation_reports/g6/8g6a ftp://data.pdbj.org/pub/pdb/validation_reports/g6/8g6a
ftp://data.pdbj.org/pub/pdb/validation_reports/g6/8g6a



 F&H Search
F&H Search Links
Links Assembly
Assembly


 Components
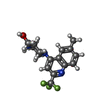
Components Homo sapiens (human) / Gene: PTPN1, PTP1B / Production host:
Homo sapiens (human) / Gene: PTPN1, PTP1B / Production host: 










 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I04 / Wavelength: 0.9795 Å
/ Beamline: I04 / Wavelength: 0.9795 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller



 PDBj
PDBj




















