+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8ek8 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human PU.1 ETS-Domain (165-270) Bound to d(AATAAAAGGAGAAGGG) | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | TRANSCRIPTION/DNA / transcription factor / protein-DNA complex / ETS family / ETS / PU.1 / TRANSCRIPTION-DNA complex | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of antifungal innate immune response / regulation of myeloid progenitor cell differentiation / anatomical structure regression / pro-T cell differentiation / positive regulation of myeloid dendritic cell chemotaxis / negative regulation of neutrophil degranulation / myeloid leukocyte differentiation / follicular B cell differentiation / positive regulation of microglial cell mediated cytotoxicity / germinal center B cell differentiation ...positive regulation of antifungal innate immune response / regulation of myeloid progenitor cell differentiation / anatomical structure regression / pro-T cell differentiation / positive regulation of myeloid dendritic cell chemotaxis / negative regulation of neutrophil degranulation / myeloid leukocyte differentiation / follicular B cell differentiation / positive regulation of microglial cell mediated cytotoxicity / germinal center B cell differentiation / TRAIL-activated apoptotic signaling pathway / granulocyte differentiation / negative regulation of MHC class II biosynthetic process / endothelial to hematopoietic transition / negative regulation of adipose tissue development / apoptotic process involved in blood vessel morphogenesis / pericyte cell differentiation / immature B cell differentiation / myeloid dendritic cell differentiation / defense response to tumor cell / oncogene-induced cell senescence / positive regulation of p38MAPK cascade / negative regulation of non-canonical NF-kappaB signal transduction / DNA-binding transcription repressor activity / positive regulation of B cell differentiation / DNA-binding transcription activator activity / STAT family protein binding / interleukin-6-mediated signaling pathway / NFAT protein binding / macrophage differentiation / cellular response to ethanol / somatic stem cell population maintenance / cis-regulatory region sequence-specific DNA binding / negative regulation of protein localization to chromatin / transforming growth factor beta receptor signaling pathway / transcription initiation-coupled chromatin remodeling / protein sequestering activity / osteoclast differentiation / lipopolysaccharide-mediated signaling pathway / negative regulation of canonical NF-kappaB signal transduction / erythrocyte differentiation / regulation of erythrocyte differentiation / positive regulation of miRNA transcription / DNA-binding transcription repressor activity, RNA polymerase II-specific / histone deacetylase binding / Transcriptional regulation of granulopoiesis / RUNX1 regulates transcription of genes involved in differentiation of HSCs / DNA-binding transcription activator activity, RNA polymerase II-specific / transcription regulator complex / sequence-specific DNA binding / DNA-binding transcription factor binding / RNA polymerase II-specific DNA-binding transcription factor binding / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / transcription cis-regulatory region binding / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / negative regulation of gene expression / negative regulation of DNA-templated transcription / chromatin binding / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / chromatin / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / RNA binding / nucleoplasm / nucleus Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | |||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.63 Å MOLECULAR REPLACEMENT / Resolution: 2.63 Å | |||||||||||||||
 Authors Authors | Terrell, J.R. / Poon, G.M.K. | |||||||||||||||
| Funding support |  United States, 4items United States, 4items
| |||||||||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2023 Journal: Cell Rep / Year: 2023Title: DNA selection by the master transcription factor PU.1. Authors: Terrell, J.R. / Taylor, S.J. / Schneider, A.L. / Lu, Y. / Vernon, T.N. / Xhani, S. / Gumpper, R.H. / Luo, M. / Wilson, W.D. / Steidl, U. / Poon, G.M.K. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8ek8.cif.gz 8ek8.cif.gz | 241.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8ek8.ent.gz pdb8ek8.ent.gz | 161.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8ek8.json.gz 8ek8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ek/8ek8 https://data.pdbj.org/pub/pdb/validation_reports/ek/8ek8 ftp://data.pdbj.org/pub/pdb/validation_reports/ek/8ek8 ftp://data.pdbj.org/pub/pdb/validation_reports/ek/8ek8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8e3kSC  8e3rC  8e4hC  8e5yC  8ebhC  8ee9C  8ej6C  8ej8C  8ek3C  8ekjC  8ekuC  8ekvC  8ekzC  8em9C  8emdC  8engC 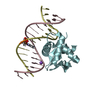 8eo1C  8eo4C  8eqgC  8eqkC  8eqlC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: DNA chain | Mass: 5053.332 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #2: DNA chain | Mass: 4436.882 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #3: Protein | Mass: 12436.583 Da / Num. of mol.: 2 / Fragment: ETS-Domain UNP residues 165-270 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SPI1 / Production host: Homo sapiens (human) / Gene: SPI1 / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.37 Å3/Da / Density % sol: 48.01 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 200 mM Ammonium Acetate, 100 mM TRIS, pH=8.5, 25% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1.00005 Å / Beamline: 5.0.2 / Wavelength: 1.00005 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Nov 10, 2021 |
| Radiation | Monochromator: Liquid nitrogen cooled dual crystal / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.00005 Å / Relative weight: 1 |
| Reflection | Resolution: 2.63→34.81 Å / Num. obs: 12962 / % possible obs: 98.71 % / Redundancy: 7 % / Biso Wilson estimate: 63.8 Å2 / CC1/2: 0.999 / CC star: 1 / Rmerge(I) obs: 0.0747 / Rpim(I) all: 0.08107 / Rrim(I) all: 0.03082 / Net I/σ(I): 17.17 |
| Reflection shell | Resolution: 2.63→2.724 Å / Redundancy: 7.1 % / Rmerge(I) obs: 0.9361 / Mean I/σ(I) obs: 2.43 / Num. unique obs: 1263 / CC1/2: 0.926 / CC star: 0.981 / Rpim(I) all: 1 / Rrim(I) all: 0.3744 / % possible all: 99.21 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 8E3K Resolution: 2.63→34.81 Å / SU ML: 0.3822 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 34.8595 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 79.42 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.63→34.81 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -21.6320871553 Å / Origin y: -0.408787658335 Å / Origin z: 53.7470606754 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller



 PDBj
PDBj






































