+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8e4k | ||||||
|---|---|---|---|---|---|---|---|
| Title | Co-crystal structure of Chaetomium glucosidase with compound 7 | ||||||
 Components Components | Chaetomium alpha glucosidase | ||||||
 Keywords Keywords | HYDROLASE/INHIBITOR / alpha glucosidase I / Hydrolase / Inhibitor complex / HYDROLASE-INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationmannosyl-oligosaccharide glucosidase / Glc3Man9GlcNAc2 oligosaccharide glucosidase activity / oligosaccharide metabolic process / protein N-linked glycosylation / endoplasmic reticulum membrane Similarity search - Function | ||||||
| Biological species |  Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Karade, S.S. / Mariuzza, R.A. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2023 Journal: J.Med.Chem. / Year: 2023Title: Structure-Based Design of Potent Iminosugar Inhibitors of Endoplasmic Reticulum alpha-Glucosidase I with Anti-SARS-CoV-2 Activity. Authors: Karade, S.S. / Franco, E.J. / Rojas, A.C. / Hanrahan, K.C. / Kolesnikov, A. / Yu, W. / MacKerell Jr., A.D. / Hill, D.C. / Weber, D.J. / Brown, A.N. / Treston, A.M. / Mariuzza, R.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8e4k.cif.gz 8e4k.cif.gz | 329.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8e4k.ent.gz pdb8e4k.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8e4k.json.gz 8e4k.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e4/8e4k https://data.pdbj.org/pub/pdb/validation_reports/e4/8e4k ftp://data.pdbj.org/pub/pdb/validation_reports/e4/8e4k ftp://data.pdbj.org/pub/pdb/validation_reports/e4/8e4k | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7r6jC  7rd2C  7revC  8e3jC  8e3pC  8e4iC  8e4zC  8e5uC  8e6gC  8ecwC  8egvC  8ehpC  8eidC  8eknC  8eleC  8epjC  8epoC  8eprC  8eq7C  8eqxC  8er4C  8etlC  8etoC  8eudC  8eurC  8eutC  8euxC  7t6wS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 3 molecules AB

| #1: Protein | Mass: 93319.812 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Thermochaetoides thermophila (fungus) / Strain: DSM 1495 / CBS 144.50 / IMI 039719 / Gene: CTHT_0061620 / Cell line (production host): Expi-HEK293 / Production host: Thermochaetoides thermophila (fungus) / Strain: DSM 1495 / CBS 144.50 / IMI 039719 / Gene: CTHT_0061620 / Cell line (production host): Expi-HEK293 / Production host:  Homo sapiens (human) / References: UniProt: G0SFD1 Homo sapiens (human) / References: UniProt: G0SFD1#5: Sugar | ChemComp-NAG / | |
|---|
-Non-polymers , 5 types, 483 molecules 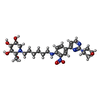








| #2: Chemical | | #3: Chemical | ChemComp-GOL / #4: Chemical | ChemComp-SO4 / #6: Chemical | ChemComp-BTB / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.89 Å3/Da / Density % sol: 57.47 % |
|---|---|
| Crystal grow | Temperature: 297 K / Method: vapor diffusion, hanging drop / pH: 6.5 / Details: 0.1 Bis Tris pH 6.5, 1.6-2.0 M Ammonium sulfate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-B / Wavelength: 1.033 Å / Beamline: 23-ID-B / Wavelength: 1.033 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Oct 4, 2021 |
| Radiation | Monochromator: Double crystal cryo-cooled Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.033 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→46.64 Å / Num. obs: 108274 / % possible obs: 95.45 % / Redundancy: 8.8 % / CC1/2: 0.992 / CC star: 0.998 / Rmerge(I) obs: 0.0995 / Rpim(I) all: 0.0353 / Net I/σ(I): 11.09 |
| Reflection shell | Resolution: 2.2→2.279 Å / Redundancy: 7.6 % / Rmerge(I) obs: 0.6227 / Mean I/σ(I) obs: 1.93 / Num. unique obs: 9443 / CC1/2: 0.861 / CC star: 0.962 / Rpim(I) all: 0.2363 / % possible all: 87.13 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 7T6W Resolution: 2.2→46.64 Å / Cor.coef. Fo:Fc: 0.958 / Cor.coef. Fo:Fc free: 0.939 / SU B: 6.145 / SU ML: 0.15 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.212 / ESU R Free: 0.187 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: U VALUES : REFINED INDIVIDUALLY
| |||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 125.46 Å2 / Biso mean: 40.982 Å2 / Biso min: 22.44 Å2
| |||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.2→46.64 Å
| |||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.2→2.257 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller



 PDBj
PDBj





