[English] 日本語
 Yorodumi
Yorodumi- PDB-8dse: Human NAMPT in complex with substrate NAM and activator quercitrin -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8dse | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human NAMPT in complex with substrate NAM and activator quercitrin | ||||||
 Components Components | Nicotinamide phosphoribosyltransferase | ||||||
 Keywords Keywords | TRANSFERASE / NAD biosynthesis enzyme:activator complex | ||||||
| Function / homology |  Function and homology information Function and homology informationnicotinamide phosphoribosyltransferase / nicotinamide phosphoribosyltransferase activity / : / NAD+ biosynthetic process / NPAS4 regulates expression of target genes / BMAL1:CLOCK,NPAS2 activates circadian expression / cytokine activity / circadian regulation of gene expression / cell junction / cell-cell signaling ...nicotinamide phosphoribosyltransferase / nicotinamide phosphoribosyltransferase activity / : / NAD+ biosynthetic process / NPAS4 regulates expression of target genes / BMAL1:CLOCK,NPAS2 activates circadian expression / cytokine activity / circadian regulation of gene expression / cell junction / cell-cell signaling / positive regulation of canonical NF-kappaB signal transduction / positive regulation of ERK1 and ERK2 cascade / nuclear speck / inflammatory response / positive regulation of cell population proliferation / positive regulation of gene expression / signal transduction / positive regulation of transcription by RNA polymerase II / extracellular exosome / identical protein binding / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.428 Å MOLECULAR REPLACEMENT / Resolution: 1.428 Å | ||||||
 Authors Authors | Ratia, K. / Xiong, R. / Shen, Z. / Thatcher, G.R. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2023 Journal: Biochemistry / Year: 2023Title: Mechanism of Allosteric Modulation of Nicotinamide Phosphoribosyltransferase to Elevate Cellular NAD. Authors: Ratia, K.M. / Shen, Z. / Gordon-Blake, J. / Lee, H. / Laham, M.S. / Krider, I.S. / Christie, N. / Ackerman-Berrier, M. / Penton, C. / Knowles, N.G. / Musku, S.R. / Fu, J. / Velma, G.R. / ...Authors: Ratia, K.M. / Shen, Z. / Gordon-Blake, J. / Lee, H. / Laham, M.S. / Krider, I.S. / Christie, N. / Ackerman-Berrier, M. / Penton, C. / Knowles, N.G. / Musku, S.R. / Fu, J. / Velma, G.R. / Xiong, R. / Thatcher, G.R.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8dse.cif.gz 8dse.cif.gz | 222.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8dse.ent.gz pdb8dse.ent.gz | 172.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8dse.json.gz 8dse.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ds/8dse https://data.pdbj.org/pub/pdb/validation_reports/ds/8dse ftp://data.pdbj.org/pub/pdb/validation_reports/ds/8dse ftp://data.pdbj.org/pub/pdb/validation_reports/ds/8dse | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8dscC  8dsdC  8dshC  8dsiC  8dtjC  2e5dS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 56666.078 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: NAMPT, PBEF, PBEF1 / Production host: Homo sapiens (human) / Gene: NAMPT, PBEF, PBEF1 / Production host:  References: UniProt: P43490, nicotinamide phosphoribosyltransferase |
|---|
-Non-polymers , 5 types, 981 molecules 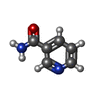


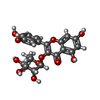





| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.36 Å3/Da / Density % sol: 47.78 % |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 0.1 M Tris-HCl, pH 8.5, 0.2 M NaCl, 20% glycerol and 13-18% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-F / Wavelength: 0.9787 Å / Beamline: 21-ID-F / Wavelength: 0.9787 Å | ||||||||||||||||||||||||||||||
| Detector | Type: RAYONIX MX-300 / Detector: CCD / Date: Jun 22, 2019 | ||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9787 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.428→19.86 Å / Num. obs: 190913 / % possible obs: 98.5 % / Redundancy: 3.5 % / CC1/2: 0.996 / Rmerge(I) obs: 0.081 / Rpim(I) all: 0.049 / Rrim(I) all: 0.095 / Net I/σ(I): 7.7 | ||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2E5D Resolution: 1.428→19.858 Å / SU ML: 0.13 / Cross valid method: THROUGHOUT / σ(F): 1.37 / Phase error: 17.92 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 57.58 Å2 / Biso mean: 18.4133 Å2 / Biso min: 7.84 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.428→19.858 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
|
 Movie
Movie Controller
Controller


 PDBj
PDBj



