+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 8bty | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of the active form of ScpB, the C5a-peptidase from Streptococcus agalactiae. | ||||||||||||
 要素 要素 | C5a peptidase | ||||||||||||
 キーワード キーワード | HYDROLASE / C5a-peptidase / virulence factor | ||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報C5a peptidase / serine-type endopeptidase activity / proteolysis / metal ion binding / membrane 類似検索 - 分子機能 | ||||||||||||
| 生物種 |  Streptococcus agalactiae (バクテリア) Streptococcus agalactiae (バクテリア) | ||||||||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 1.7 Å 分子置換 / 解像度: 1.7 Å | ||||||||||||
 データ登録者 データ登録者 | Kagawa, T.F. / Cooney, J.C. / Miclot, T. / Cullen, R. | ||||||||||||
| 資金援助 |  アイルランド, 3件 アイルランド, 3件
| ||||||||||||
 引用 引用 |  ジャーナル: Proteins / 年: 2024 ジャーナル: Proteins / 年: 2024タイトル: The 1.7 angstrom crystal structure of the C5a peptidase from Streptococcus agalactiae (ScpB) reveals an active site competent for catalysis. 著者: Cullen, R. / Tecza, M. / Miclot, T. / Behan, S. / Jain, M. / Avink, M.K. / Cooney, J.C. / Kagawa, T.F. #1:  ジャーナル: Proc Natl Acad Sci U S A / 年: 2005 ジャーナル: Proc Natl Acad Sci U S A / 年: 2005タイトル: Structure of the streptococcal cell wall C5a peptidase. 著者: Brown, C.K. / Gu, Z.Y. / Matsuka, Y.V. / Purushothaman, S.S. / Winter, L.A. / Cleary, P.P. / Olmsted, S.B. / Ohlendorf, D.H. / Earhart, C.A. | ||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  8bty.cif.gz 8bty.cif.gz | 480.4 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb8bty.ent.gz pdb8bty.ent.gz | 313.8 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  8bty.json.gz 8bty.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  8bty_validation.pdf.gz 8bty_validation.pdf.gz | 476.8 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  8bty_full_validation.pdf.gz 8bty_full_validation.pdf.gz | 482.8 KB | 表示 | |
| XML形式データ |  8bty_validation.xml.gz 8bty_validation.xml.gz | 39.1 KB | 表示 | |
| CIF形式データ |  8bty_validation.cif.gz 8bty_validation.cif.gz | 59.6 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/bt/8bty https://data.pdbj.org/pub/pdb/validation_reports/bt/8bty ftp://data.pdbj.org/pub/pdb/validation_reports/bt/8bty ftp://data.pdbj.org/pub/pdb/validation_reports/bt/8bty | HTTPS FTP |
-関連構造データ
| 関連構造データ |  3eifS S: 精密化の開始モデル |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
| 実験データセット #1 | データ参照:  10.51093/xrd-00106 / データの種類: diffraction image data 10.51093/xrd-00106 / データの種類: diffraction image data |
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| 単位格子 |
|
- 要素
要素
-タンパク質 , 1種, 2分子 AB
| #1: タンパク質 | 分子量: 109862.766 Da / 分子数: 2 / 由来タイプ: 組換発現 詳細: Sample sequence begins with residue N31. Chain B is likely a peptide produced by auto-proteolytic activation of the enzyme. Residues in this chain are in the pro-peptide region. Assignments ...詳細: Sample sequence begins with residue N31. Chain B is likely a peptide produced by auto-proteolytic activation of the enzyme. Residues in this chain are in the pro-peptide region. Assignments of density for A84, P85, Q86, and A87 in Chain B are tentative. 由来: (組換発現)  Streptococcus agalactiae (バクテリア) Streptococcus agalactiae (バクテリア)遺伝子: scpB / 発現宿主:  |
|---|
-非ポリマー , 6種, 649分子 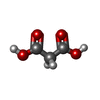










| #2: 化合物 | ChemComp-MLA / | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #3: 化合物 | ChemComp-SO4 / #4: 化合物 | ChemComp-CA / | #5: 化合物 | ChemComp-NA / | #6: 化合物 | ChemComp-EPE / | #7: 水 | ChemComp-HOH / | |
-詳細
| 研究の焦点であるリガンドがあるか | N |
|---|
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 2.53 Å3/Da / 溶媒含有率: 51.5 % |
|---|---|
| 結晶化 | 温度: 293 K / 手法: 蒸気拡散法, ハンギングドロップ法 / pH: 7.8 / 詳細: 2.5 M ammonium sulfate, 0.1 M Hepes/KOH pH 7.8 |
-データ収集
| 回折 | 平均測定温度: 100 K / Serial crystal experiment: N |
|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  Diamond Diamond  / ビームライン: I04 / 波長: 0.9795 Å / ビームライン: I04 / 波長: 0.9795 Å |
| 検出器 | タイプ: DECTRIS PILATUS 6M / 検出器: PIXEL / 日付: 2016年12月15日 |
| 放射 | プロトコル: SINGLE WAVELENGTH / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
| 放射波長 | 波長: 0.9795 Å / 相対比: 1 |
| 反射 | 解像度: 1.7→48.253 Å / Num. obs: 127120 / % possible obs: 99.88 % / 冗長度: 40 % / Biso Wilson estimate: 33.63 Å2 / CC1/2: 1 / CC star: 1 / Rpim(I) all: 0.0334 / Rrim(I) all: 0.2124 / Net I/σ(I): 16.63 |
| 反射 シェル | 解像度: 1.7→1.761 Å / 冗長度: 39.6 % / Mean I/σ(I) obs: 0.34 / Num. unique obs: 12428 / CC1/2: 0.237 / CC star: 0.619 / Rpim(I) all: 0.8948 / Rrim(I) all: 5.659 / % possible all: 99.01 |
- 解析
解析
| ソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  分子置換 分子置換開始モデル: 3EIF 解像度: 1.7→44.84 Å / SU ML: 0.3451 / 交差検証法: FREE R-VALUE / σ(F): 1.33 / 位相誤差: 26.0543 立体化学のターゲット値: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶媒の処理 | 減衰半径: 1.1 Å / VDWプローブ半径: 1.2 Å / 溶媒モデル: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso mean: 37.65 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 1.7→44.84 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS精密化 シェル |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化 TLS | 手法: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化 TLSグループ | Refine-ID: X-RAY DIFFRACTION / Auth asym-ID: A / Label asym-ID: A
|
 ムービー
ムービー コントローラー
コントローラー



 PDBj
PDBj








