[English] 日本語
 Yorodumi
Yorodumi- PDB-8abt: Crystal structure of NaLdpA in complex with the product analog Re... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8abt | ||||||
|---|---|---|---|---|---|---|---|
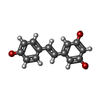
| Title | Crystal structure of NaLdpA in complex with the product analog Resveratrol | ||||||
 Components Components | SnoaL-like domain-containing protein | ||||||
 Keywords Keywords | LYASE / lignin / dehydratase | ||||||
| Function / homology | SnoaL-like domain / SnoaL-like domain / NTF2-like domain superfamily / RESVERATROL / SnoaL-like domain-containing protein Function and homology information Function and homology information | ||||||
| Biological species |  Novosphingobium aromaticivorans DSM 12444 (bacteria) Novosphingobium aromaticivorans DSM 12444 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.39 Å SAD / Resolution: 1.39 Å | ||||||
 Authors Authors | Zahn, M. / Kuatsjah, E. / Beckham, G.T. / McGeehan, J.E. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2023 Journal: Proc.Natl.Acad.Sci.USA / Year: 2023Title: Biochemical and structural characterization of a sphingomonad diarylpropane lyase for cofactorless deformylation. Authors: Kuatsjah, E. / Zahn, M. / Chen, X. / Kato, R. / Hinchen, D.J. / Konev, M.O. / Katahira, R. / Orr, C. / Wagner, A. / Zou, Y. / Haugen, S.J. / Ramirez, K.J. / Michener, J.K. / Pickford, A.R. / ...Authors: Kuatsjah, E. / Zahn, M. / Chen, X. / Kato, R. / Hinchen, D.J. / Konev, M.O. / Katahira, R. / Orr, C. / Wagner, A. / Zou, Y. / Haugen, S.J. / Ramirez, K.J. / Michener, J.K. / Pickford, A.R. / Kamimura, N. / Masai, E. / Houk, K.N. / McGeehan, J.E. / Beckham, G.T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8abt.cif.gz 8abt.cif.gz | 326.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8abt.ent.gz pdb8abt.ent.gz | 258 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8abt.json.gz 8abt.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8abt_validation.pdf.gz 8abt_validation.pdf.gz | 482 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8abt_full_validation.pdf.gz 8abt_full_validation.pdf.gz | 486 KB | Display | |
| Data in XML |  8abt_validation.xml.gz 8abt_validation.xml.gz | 11.7 KB | Display | |
| Data in CIF |  8abt_validation.cif.gz 8abt_validation.cif.gz | 19.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ab/8abt https://data.pdbj.org/pub/pdb/validation_reports/ab/8abt ftp://data.pdbj.org/pub/pdb/validation_reports/ab/8abt ftp://data.pdbj.org/pub/pdb/validation_reports/ab/8abt | HTTPS FTP |
-Related structure data
| Related structure data |  8abuC  8abvC  8abwC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||
| Components on special symmetry positions |
| |||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1 / Beg auth comp-ID: ALA / Beg label comp-ID: ALA / End auth comp-ID: GLN / End label comp-ID: GLN / Auth seq-ID: 6 - 239 / Label seq-ID: 6 - 239
NCS ensembles : (Details: Local NCS retraints between domains: 1 2) |
- Components
Components
| #1: Protein | Mass: 28830.535 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Novosphingobium aromaticivorans DSM 12444 (bacteria) Novosphingobium aromaticivorans DSM 12444 (bacteria)Strain: ATCC 700278 / DSM 12444 / CCUG 56034 / CIP 105152 / NBRC 16084 / F199 Gene: Saro_2805 / Production host:  #2: Chemical | #3: Chemical | ChemComp-SO4 / #4: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.6 Å3/Da / Density % sol: 52.67 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 2 M ammonium sulfate, 2% PEG 400, 0.1 M HEPES pH 7.5 |
-Data collection
| Diffraction |
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| |||||||||||||||||||||||||||
| Detector |
| |||||||||||||||||||||||||||
| Radiation |
| |||||||||||||||||||||||||||
| Radiation wavelength |
| |||||||||||||||||||||||||||
| Reflection | Entry-ID: 8ABT / % possible obs: 100 %
| |||||||||||||||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.39→51.568 Å / Cor.coef. Fo:Fc: 0.94 / Cor.coef. Fo:Fc free: 0.936 / WRfactor Rfree: 0.238 / WRfactor Rwork: 0.221 / SU B: 4.592 / SU ML: 0.085 / Average fsc free: 0.7679 / Average fsc work: 0.7811 / Cross valid method: FREE R-VALUE / ESU R: 0.075 / ESU R Free: 0.074 SAD / Resolution: 1.39→51.568 Å / Cor.coef. Fo:Fc: 0.94 / Cor.coef. Fo:Fc free: 0.936 / WRfactor Rfree: 0.238 / WRfactor Rwork: 0.221 / SU B: 4.592 / SU ML: 0.085 / Average fsc free: 0.7679 / Average fsc work: 0.7811 / Cross valid method: FREE R-VALUE / ESU R: 0.075 / ESU R Free: 0.074 Details: Hydrogens have been added in their riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK BULK SOLVENT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 18.765 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.39→51.568 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj