[English] 日本語
 Yorodumi
Yorodumi- PDB-8a9n: Structure of DpA polyamine acetyltransferase in complex with 1,3-DAP -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8a9n | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of DpA polyamine acetyltransferase in complex with 1,3-DAP | ||||||
 Components Components | Acetyltransferase | ||||||
 Keywords Keywords | TRANSFERASE / polyamine acetyltransferase / GNAT / bacterial biofilm formation / Acinetorbacter baumannii | ||||||
| Function / homology |  Function and homology information Function and homology informationacyltransferase activity, transferring groups other than amino-acyl groups / metal ion binding / membrane Similarity search - Function | ||||||
| Biological species |  Acinetobacter baumannii (bacteria) Acinetobacter baumannii (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.854 Å MOLECULAR REPLACEMENT / Resolution: 1.854 Å | ||||||
 Authors Authors | Garcia-Pino, A. / Jurenas, D. | ||||||
| Funding support |  Belgium, 1items Belgium, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A polyamine acetyltransferase regulates the motility and biofilm formation of Acinetobacter baumannii. Authors: Armalyte, J. / Cepauskas, A. / Sakalyte, G. / Martinkus, J. / Skerniskyte, J. / Martens, C. / Suziedeliene, E. / Garcia-Pino, A. / Jurenas, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8a9n.cif.gz 8a9n.cif.gz | 150 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8a9n.ent.gz pdb8a9n.ent.gz | 116.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8a9n.json.gz 8a9n.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/a9/8a9n https://data.pdbj.org/pub/pdb/validation_reports/a9/8a9n ftp://data.pdbj.org/pub/pdb/validation_reports/a9/8a9n ftp://data.pdbj.org/pub/pdb/validation_reports/a9/8a9n | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8a9oC  6gtpS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 18337.783 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Acinetobacter baumannii (bacteria) Acinetobacter baumannii (bacteria)Gene: Aba9201_01540, ABR2091_2278, ABUW_1603, ACX61_07190, APC21_20150, APD31_09770, AYR68_11090, B7L45_07990, BAA1790NC_2350, BAA1790NC_2370, BS065_06845, C2U32_07150, C6N18_11935, CBL15_06645, ...Gene: Aba9201_01540, ABR2091_2278, ABUW_1603, ACX61_07190, APC21_20150, APD31_09770, AYR68_11090, B7L45_07990, BAA1790NC_2350, BAA1790NC_2370, BS065_06845, C2U32_07150, C6N18_11935, CBL15_06645, CPI82_05975, CSB70_2184, CTZ19_07130, D8O08_013765, DLI71_04685, DLI72_12170, DVA69_08365, E1A87_13275, E2532_16715, E2533_09885, E2534_09490, E2535_09465, E2536_09480, E2538_08415, E2539_09380, E2540_11625, E2541_08395, EA686_20485, EA720_017220, EA722_17035, EGM95_07835, EKS29_07930, EWO96_04140, F2P40_03430, F4T85_15855, FDN00_08240, FE003_07065, FGL68_01250, FJU42_16945, FJU76_02830, GNY86_02530, GSE42_12350, H0529_02675, H1058_07095, HB367_07820, HBK86_10405, IAG11_07575, IMO23_11370, ITE13_15710, NCTC13305_03823, NCTC13421_01499, SAMEA104305318_03080, SAMEA104305340_02732, SAMEA104305385_00035, SI89_01875 Production host:  References: UniProt: V5VBK4 |
|---|
-Non-polymers , 6 types, 243 molecules 

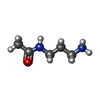








| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-LFU / ~{ | #5: Chemical | ChemComp-GOL / | #6: Chemical | ChemComp-13D / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.17 Å3/Da / Density % sol: 43.34 % |
|---|---|
| Crystal grow | Temperature: 293.16 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: 0.1M TRIS hydrochloride pH 8.5, 30% w/v Polyethylene glycol 4,000, 0.1 mM acetyl-CoA |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 2 / Wavelength: 0.9801 Å / Beamline: PROXIMA 2 / Wavelength: 0.9801 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Jun 3, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9801 Å / Relative weight: 1 |
| Reflection | Resolution: 1.85→41.66 Å / Num. obs: 25133 / % possible obs: 97.37 % / Redundancy: 4.6 % / Biso Wilson estimate: 38.49 Å2 / CC1/2: 0.996 / Rpim(I) all: 0.054 / Net I/σ(I): 8.43 |
| Reflection shell | Resolution: 1.85→1.97 Å / Num. unique obs: 2407 / CC1/2: 0.418 / Rpim(I) all: 0.67 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6GTP Resolution: 1.854→41.66 Å / Cor.coef. Fo:Fc: 0.936 / Cor.coef. Fo:Fc free: 0.92 / SU R Cruickshank DPI: 0.177 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.183 / SU Rfree Blow DPI: 0.16 / SU Rfree Cruickshank DPI: 0.158
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 36.13 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.25 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.854→41.66 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.854→1.87 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj










