[English] 日本語
 Yorodumi
Yorodumi- PDB-7zed: Solution structure of thanatin-like derivative 7 in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7zed | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Solution structure of thanatin-like derivative 7 in complex with E.coli LptA mutant Q62L | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | ANTIBIOTIC / STRUCTURE FROM CYANA 3.98.11 | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationglycolipid transfer activity / transporter complex / lipopolysaccharide transport / Gram-negative-bacterium-type cell outer membrane assembly / defense response to fungus / cell outer membrane / lipopolysaccharide binding / outer membrane-bounded periplasmic space / killing of cells of another organism / periplasmic space ...glycolipid transfer activity / transporter complex / lipopolysaccharide transport / Gram-negative-bacterium-type cell outer membrane assembly / defense response to fungus / cell outer membrane / lipopolysaccharide binding / outer membrane-bounded periplasmic space / killing of cells of another organism / periplasmic space / defense response to bacterium / innate immune response / extracellular region / identical protein binding Similarity search - Function | ||||||||||||
| Biological species |   Podisus maculiventris (spined soldier bug) Podisus maculiventris (spined soldier bug) | ||||||||||||
| Method | SOLUTION NMR / na | ||||||||||||
 Authors Authors | Moehle, K. / Zerbe, O. | ||||||||||||
| Funding support | European Union, 1items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Peptidomimetic antibiotics disrupt the lipopolysaccharide transport bridge of drug-resistant Enterobacteriaceae. Authors: Schuster, M. / Brabet, E. / Oi, K.K. / Desjonqueres, N. / Moehle, K. / Le Poupon, K. / Hell, S. / Gable, S. / Rithie, V. / Dillinger, S. / Zbinden, P. / Luther, A. / Li, C. / Stiegeler, S. / ...Authors: Schuster, M. / Brabet, E. / Oi, K.K. / Desjonqueres, N. / Moehle, K. / Le Poupon, K. / Hell, S. / Gable, S. / Rithie, V. / Dillinger, S. / Zbinden, P. / Luther, A. / Li, C. / Stiegeler, S. / D'Arco, C. / Locher, H. / Remus, T. / DiMaio, S. / Motta, P. / Wach, A. / Jung, F. / Upert, G. / Obrecht, D. / Benghezal, M. / Zerbe, O. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7zed.cif.gz 7zed.cif.gz | 808.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7zed.ent.gz pdb7zed.ent.gz | 679.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7zed.json.gz 7zed.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ze/7zed https://data.pdbj.org/pub/pdb/validation_reports/ze/7zed ftp://data.pdbj.org/pub/pdb/validation_reports/ze/7zed ftp://data.pdbj.org/pub/pdb/validation_reports/ze/7zed | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7qs6C  7zaxC  8bssC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 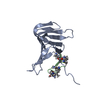
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 12853.328 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: The sequence doesnt contain the signaling sequence (Residues 1-27 from UNIPROT entry P0ADV1). The first residue is numbered 28. The expressed construct is 28-159. Deposited are coordinates ...Details: The sequence doesnt contain the signaling sequence (Residues 1-27 from UNIPROT entry P0ADV1). The first residue is numbered 28. The expressed construct is 28-159. Deposited are coordinates for the structured part containing residues 28-145. Source: (gene. exp.)   |
|---|---|
| #2: Protein/peptide | Mass: 2007.387 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)  Podisus maculiventris (spined soldier bug) / References: UniProt: P55788 Podisus maculiventris (spined soldier bug) / References: UniProt: P55788 |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Type: solution Contents: 300 uM [U-13C; U-15N] Lipopolysaccharide export system protein LptA, 20 mM sodium phosphate, 150 mM sodium chloride, 90% H2O/10% D2O Details: 13C,15N-labelled LptA-Q62L (28-145) / Label: LptAm_15N13C / Solvent system: 90% H2O/10% D2O | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||||||
| Sample conditions | Ionic strength: 150 mM / Ionic strength err: 10 / Label: conditions_1 / pH: 7 / PH err: 0.1 / Pressure: 1 atm / Pressure err: 0.01 / Temperature: 298 K / Temperature err: 0.2 |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: na / Software ordinal: 1 | ||||||||||||||||||||||||
| NMR representative | Selection criteria: target function | ||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 100 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller


 PDBj
PDBj HSQC
HSQC