[English] 日本語
 Yorodumi
Yorodumi- PDB-7xma: Crystal structure of Bovine heart cytochrome c oxidase, apo struc... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7xma | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Bovine heart cytochrome c oxidase, apo structure with DMSO | ||||||
 Components Components | (Cytochrome c oxidase subunit ...) x 13 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / respiratory enzyme / membrane protein / heme protein / apo structure | ||||||
| Function / homology |  Function and homology information Function and homology information: / Complex IV assembly / TP53 Regulates Metabolic Genes / respiratory chain complex IV assembly / Cytoprotection by HMOX1 / mitochondrial respirasome assembly / Respiratory electron transport / respiratory chain complex IV / respiratory chain complex / cytochrome-c oxidase ...: / Complex IV assembly / TP53 Regulates Metabolic Genes / respiratory chain complex IV assembly / Cytoprotection by HMOX1 / mitochondrial respirasome assembly / Respiratory electron transport / respiratory chain complex IV / respiratory chain complex / cytochrome-c oxidase / oxidative phosphorylation / mitochondrial electron transport, cytochrome c to oxygen / cytochrome-c oxidase activity / Mitochondrial protein degradation / electron transport coupled proton transport / ATP synthesis coupled electron transport / enzyme regulator activity / aerobic respiration / central nervous system development / respiratory electron transport chain / oxidoreductase activity / mitochondrial inner membrane / copper ion binding / heme binding / mitochondrion / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.2 Å MOLECULAR REPLACEMENT / Resolution: 2.2 Å | ||||||
 Authors Authors | Nishida, Y. / Shinzawa-Itoh, K. / Mizuno, N. / Kumasaka, T. / Yoshikawa, S. / Tsukihara, T. / Takashima, S. / Shintani, Y. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Identifying antibiotics based on structural differences in the conserved allostery from mitochondrial heme-copper oxidases. Authors: Yuya Nishida / Sachiko Yanagisawa / Rikuri Morita / Hideki Shigematsu / Kyoko Shinzawa-Itoh / Hitomi Yuki / Satoshi Ogasawara / Ken Shimuta / Takashi Iwamoto / Chisa Nakabayashi / Waka ...Authors: Yuya Nishida / Sachiko Yanagisawa / Rikuri Morita / Hideki Shigematsu / Kyoko Shinzawa-Itoh / Hitomi Yuki / Satoshi Ogasawara / Ken Shimuta / Takashi Iwamoto / Chisa Nakabayashi / Waka Matsumura / Hisakazu Kato / Chai Gopalasingam / Takemasa Nagao / Tasneem Qaqorh / Yusuke Takahashi / Satoru Yamazaki / Katsumasa Kamiya / Ryuhei Harada / Nobuhiro Mizuno / Hideyuki Takahashi / Yukihiro Akeda / Makoto Ohnishi / Yoshikazu Ishii / Takashi Kumasaka / Takeshi Murata / Kazumasa Muramoto / Takehiko Tosha / Yoshitsugu Shiro / Teruki Honma / Yasuteru Shigeta / Minoru Kubo / Seiji Takashima / Yasunori Shintani /  Abstract: Antimicrobial resistance (AMR) is a global health problem. Despite the enormous efforts made in the last decade, threats from some species, including drug-resistant Neisseria gonorrhoeae, continue to ...Antimicrobial resistance (AMR) is a global health problem. Despite the enormous efforts made in the last decade, threats from some species, including drug-resistant Neisseria gonorrhoeae, continue to rise and would become untreatable. The development of antibiotics with a different mechanism of action is seriously required. Here, we identified an allosteric inhibitory site buried inside eukaryotic mitochondrial heme-copper oxidases (HCOs), the essential respiratory enzymes for life. The steric conformation around the binding pocket of HCOs is highly conserved among bacteria and eukaryotes, yet the latter has an extra helix. This structural difference in the conserved allostery enabled us to rationally identify bacterial HCO-specific inhibitors: an antibiotic compound against ceftriaxone-resistant Neisseria gonorrhoeae. Molecular dynamics combined with resonance Raman spectroscopy and stopped-flow spectroscopy revealed an allosteric obstruction in the substrate accessing channel as a mechanism of inhibition. Our approach opens fresh avenues in modulating protein functions and broadens our options to overcome AMR. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7xma.cif.gz 7xma.cif.gz | 1.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7xma.ent.gz pdb7xma.ent.gz | 964 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7xma.json.gz 7xma.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7xma_validation.pdf.gz 7xma_validation.pdf.gz | 8.9 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7xma_full_validation.pdf.gz 7xma_full_validation.pdf.gz | 9.2 MB | Display | |
| Data in XML |  7xma_validation.xml.gz 7xma_validation.xml.gz | 180.7 KB | Display | |
| Data in CIF |  7xma_validation.cif.gz 7xma_validation.cif.gz | 238.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xm/7xma https://data.pdbj.org/pub/pdb/validation_reports/xm/7xma ftp://data.pdbj.org/pub/pdb/validation_reports/xm/7xma ftp://data.pdbj.org/pub/pdb/validation_reports/xm/7xma | HTTPS FTP |
-Related structure data
| Related structure data |  7xmbC  7xmcC  7xmdC  5b1aS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
|
- Components
Components
-Cytochrome c oxidase subunit ... , 13 types, 26 molecules ANBOCPDQERFSGTHUIVJWKXLYMZ
| #1: Protein | Mass: 57093.852 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #2: Protein | Mass: 65935.945 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P68530, UniProt: Q6EMS9, cytochrome-c oxidase #3: Protein | Mass: 29971.611 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #4: Protein | Mass: 17179.646 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #5: Protein | Mass: 12453.081 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #6: Protein | Mass: 10684.038 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #7: Protein | Mass: 9532.667 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #8: Protein | Mass: 10039.244 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #9: Protein | Mass: 8494.982 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #10: Protein | Mass: 6682.726 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #11: Protein | Mass: 6365.217 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #12: Protein/peptide | Mass: 5449.396 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  #13: Protein/peptide | Mass: 4967.756 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Sugars , 1 types, 4 molecules 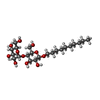
| #27: Sugar | ChemComp-DMU / |
|---|
-Non-polymers , 14 types, 2534 molecules 


























| #14: Chemical | ChemComp-HEA / #15: Chemical | #16: Chemical | #17: Chemical | #18: Chemical | #19: Chemical | ChemComp-PGV / ( #20: Chemical | ChemComp-TGL / #21: Chemical | #22: Chemical | ChemComp-CDL / #23: Chemical | ChemComp-CHD / #24: Chemical | ChemComp-PEK / ( #25: Chemical | #26: Chemical | #28: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.36 Å3/Da / Density % sol: 63.4 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: batch mode / pH: 6.8 Details: sodium phosphate, PEG4000, decyl maltoside, ethylene glycol, DMSO |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL26B1 / Wavelength: 1 Å / Beamline: BL26B1 / Wavelength: 1 Å |
| Detector | Type: RAYONIX MX225HE / Detector: CCD / Date: Nov 4, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→29.97 Å / Num. obs: 644464 / % possible obs: 99.4 % / Redundancy: 3.87 % / Biso Wilson estimate: 30.02 Å2 / CC1/2: 0.998 / Rmerge(I) obs: 0.069 / Rpim(I) all: 0.07 / Net I/σ(I): 15.96 |
| Reflection shell | Resolution: 2.2→2.33 Å / Redundancy: 3.68 % / Rmerge(I) obs: 0.327 / Mean I/σ(I) obs: 4.36 / Num. unique obs: 103395 / CC1/2: 0.938 / Rpim(I) all: 0.385 / Rrim(I) all: 0.327 / % possible all: 99.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5b1a Resolution: 2.2→29.97 Å / SU ML: 0.2296 / Cross valid method: FREE R-VALUE / σ(F): 1.09 / Phase error: 24.8785 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.37 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→29.97 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj


















