[English] 日本語
 Yorodumi
Yorodumi- PDB-7x3m: Crystal structure of Aldo-keto reductase 1C3 complexed with compo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7x3m | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Aldo-keto reductase 1C3 complexed with compound S07045 | ||||||
 Components Components | Aldo-keto reductase family 1 member C3 | ||||||
 Keywords Keywords | OXIDOREDUCTASE / Lipid metabolism / NAD / NADP | ||||||
| Function / homology |  Function and homology information Function and homology informationprostaglandin-F synthase / testosterone 17beta-dehydrogenase (NADP+) / prostaglandin D2 11-ketoreductase activity / ketoreductase activity / prostaglandin F synthase activity / cellular response to prostaglandin stimulus / cellular response to corticosteroid stimulus / macromolecule metabolic process / 15-hydroxyprostaglandin-D dehydrogenase (NADP+) activity / 3beta(or 20alpha)-hydroxysteroid dehydrogenase ...prostaglandin-F synthase / testosterone 17beta-dehydrogenase (NADP+) / prostaglandin D2 11-ketoreductase activity / ketoreductase activity / prostaglandin F synthase activity / cellular response to prostaglandin stimulus / cellular response to corticosteroid stimulus / macromolecule metabolic process / 15-hydroxyprostaglandin-D dehydrogenase (NADP+) activity / 3beta(or 20alpha)-hydroxysteroid dehydrogenase / negative regulation of retinoic acid biosynthetic process / 5-alpha-androstane-3-beta,17-beta-diol dehydrogenase (NADP+) activity / Delta4-3-oxosteroid 5beta-reductase activity / farnesol catabolic process / geranylgeranyl reductase activity / 3alpha-hydroxysteroid 3-dehydrogenase / cellular response to jasmonic acid stimulus / 3alpha(17beta)-hydroxysteroid dehydrogenase (NAD+) / prostanoid biosynthetic process / testosterone dehydrogenase (NADP+) activity / androsterone dehydrogenase [NAD(P)+] activity / ketosteroid monooxygenase activity / regulation of testosterone biosynthetic process / RA biosynthesis pathway / testosterone biosynthetic process / : / 3alpha(or 20beta)-hydroxysteroid dehydrogenase / androstan-3-alpha,17-beta-diol dehydrogenase (NAD+) activity / Synthesis of bile acids and bile salts via 24-hydroxycholesterol / testosterone dehydrogenase (NAD+) activity / regulation of retinoic acid receptor signaling pathway / cellular response to prostaglandin D stimulus / progesterone metabolic process / 17beta-estradiol 17-dehydrogenase / retinal metabolic process / Synthesis of Prostaglandins (PG) and Thromboxanes (TX) / estradiol 17-beta-dehydrogenase [NAD(P)+] activity / all-trans-retinol dehydrogenase (NAD+) activity / : / prostaglandin H2 endoperoxidase reductase activity / all-trans-retinol dehydrogenase (NADP+) activity / Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor / bile acid binding / daunorubicin metabolic process / doxorubicin metabolic process / retinal dehydrogenase (NAD+) activity / aldose reductase (NADPH) activity / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / prostaglandin metabolic process / renal absorption / steroid metabolic process / positive regulation of endothelial cell apoptotic process / Synthesis of bile acids and bile salts via 27-hydroxycholesterol / Synthesis of bile acids and bile salts via 7alpha-hydroxycholesterol / retinoid metabolic process / Retinoid metabolism and transport / keratinocyte differentiation / response to nutrient / cellular response to calcium ion / cellular response to starvation / male gonad development / positive regulation of reactive oxygen species metabolic process / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / G protein-coupled receptor signaling pathway / positive regulation of cell population proliferation / extracellular exosome / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.694 Å MOLECULAR REPLACEMENT / Resolution: 2.694 Å | ||||||
 Authors Authors | Jiang, J. / Liu, Y. / He, S. / Chen, Y. / Chu, X. / Liu, Y. / Guo, Q. / Zhao, L. / Feng, F. / Liu, W. ...Jiang, J. / Liu, Y. / He, S. / Chen, Y. / Chu, X. / Liu, Y. / Guo, Q. / Zhao, L. / Feng, F. / Liu, W. / Zhang, X. / Fang, P. / Sun, H. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2023 Journal: J.Med.Chem. / Year: 2023Title: Development of Biaryl-Containing Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors for Reversing AKR1C3-Mediated Drug Resistance in Cancer Treatment. Authors: He, S. / Chu, X. / Wu, Y. / Jiang, J. / Fang, P. / Chen, Y. / Liu, Y. / Qiu, Z. / Xiao, Y. / Li, Z. / Pan, D. / Zhang, Q. / Xie, H. / Xing, S. / Feng, F. / Liu, W. / Guo, Q. / Zhao, L. / Yang, P. / Sun, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7x3m.cif.gz 7x3m.cif.gz | 267.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7x3m.ent.gz pdb7x3m.ent.gz | 213.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7x3m.json.gz 7x3m.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x3/7x3m https://data.pdbj.org/pub/pdb/validation_reports/x3/7x3m ftp://data.pdbj.org/pub/pdb/validation_reports/x3/7x3m ftp://data.pdbj.org/pub/pdb/validation_reports/x3/7x3m | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7x3lC 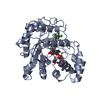 8i0cC  7wqmS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 37735.113 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: AKR1C3, DDH1, HSD17B5, KIAA0119, PGFS / Production host: Homo sapiens (human) / Gene: AKR1C3, DDH1, HSD17B5, KIAA0119, PGFS / Production host:  References: UniProt: P42330, Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor, 3beta(or 20alpha)-hydroxysteroid dehydrogenase, 3alpha(or 20beta)-hydroxysteroid ...References: UniProt: P42330, Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor, 3beta(or 20alpha)-hydroxysteroid dehydrogenase, 3alpha(or 20beta)-hydroxysteroid dehydrogenase, 17beta-estradiol 17-dehydrogenase, 3alpha-hydroxysteroid 3-dehydrogenase, prostaglandin-F synthase, 3alpha(17beta)-hydroxysteroid dehydrogenase (NAD+), testosterone 17beta-dehydrogenase (NADP+) #2: Chemical | #3: Chemical | #4: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.52 Å3/Da / Density % sol: 51.25 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 6 / Details: MES pH 6.0, Ammonium chloride, PEG 6000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL17U1 / Wavelength: 0.9791 Å / Beamline: BL17U1 / Wavelength: 0.9791 Å | ||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS EIGER2 S 9M / Detector: PIXEL / Date: Nov 8, 2021 | ||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9791 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.69→60.38 Å / Num. obs: 17327 / % possible obs: 84 % / Redundancy: 2 % / Biso Wilson estimate: 25.47 Å2 / CC1/2: 0.879 / Rmerge(I) obs: 0.106 / Rpim(I) all: 0.092 / Rrim(I) all: 0.141 / Net I/σ(I): 6.1 / Num. measured all: 35235 | ||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 7WQM Resolution: 2.694→31.697 Å / SU ML: 0.28 / Cross valid method: THROUGHOUT / σ(F): 1.35 / Phase error: 23.61 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 95.19 Å2 / Biso mean: 31.9353 Å2 / Biso min: 13.18 Å2 | ||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.694→31.697 Å
| ||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
| ||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 17.5396 Å / Origin y: -10.5133 Å / Origin z: 21.2571 Å
| ||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj