[English] 日本語
 Yorodumi
Yorodumi- PDB-7w3l: Crystal structure of LSD1 in complex with cis-4-Br-2,5-F2-PCPA (S1024) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7w3l | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of LSD1 in complex with cis-4-Br-2,5-F2-PCPA (S1024) | ||||||
 Components Components | Lysine-specific histone demethylase 1A | ||||||
 Keywords Keywords | OXIDOREDUCTASE / DEMETHYLASE / AMINE OXIDASE / CHROMATIN / HISTONE / FAD / MECHANISM-BASED INHIBITOR | ||||||
| Function / homology |  Function and homology information Function and homology informationguanine metabolic process / negative regulation of transcription initiation-coupled chromatin remodeling / protein demethylase activity / [histone H3]-N6,N6-dimethyl-L-lysine4 FAD-dependent demethylase / FAD-dependent H3K4me/H3K4me3 demethylase activity / histone H3K4 demethylase activity / telomeric repeat-containing RNA binding / neuron maturation / muscle cell development / positive regulation of neural precursor cell proliferation ...guanine metabolic process / negative regulation of transcription initiation-coupled chromatin remodeling / protein demethylase activity / [histone H3]-N6,N6-dimethyl-L-lysine4 FAD-dependent demethylase / FAD-dependent H3K4me/H3K4me3 demethylase activity / histone H3K4 demethylase activity / telomeric repeat-containing RNA binding / neuron maturation / muscle cell development / positive regulation of neural precursor cell proliferation / negative regulation of intrinsic apoptotic signaling pathway by p53 class mediator / MRF binding / regulation of androgen receptor signaling pathway / DNA repair complex / nuclear androgen receptor binding / negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / regulation of double-strand break repair via homologous recombination / positive regulation of neuroblast proliferation / DNA repair-dependent chromatin remodeling / positive regulation of stem cell proliferation / histone H3K9 demethylase activity / negative regulation of DNA damage response, signal transduction by p53 class mediator / histone methyltransferase complex / histone demethylase activity / positive regulation of cell size / positive regulation of epithelial to mesenchymal transition / response to fungicide / NR1H3 & NR1H2 regulate gene expression linked to cholesterol transport and efflux / epigenetic regulation of gene expression / cellular response to cAMP / Regulation of PTEN gene transcription / positive regulation of protein ubiquitination / HDACs deacetylate histones / promoter-specific chromatin binding / positive regulation of neuron projection development / cellular response to gamma radiation / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / HDMs demethylate histones / cerebral cortex development / protein modification process / p53 binding / cellular response to UV / transcription corepressor activity / flavin adenine dinucleotide binding / regulation of protein localization / positive regulation of cold-induced thermogenesis / Factors involved in megakaryocyte development and platelet production / transcription regulator complex / Estrogen-dependent gene expression / DNA-binding transcription factor binding / Potential therapeutics for SARS / RNA polymerase II-specific DNA-binding transcription factor binding / transcription coactivator activity / oxidoreductase activity / chromosome, telomeric region / chromatin binding / regulation of transcription by RNA polymerase II / chromatin / enzyme binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / protein-containing complex / nucleoplasm / identical protein binding / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.51 Å MOLECULAR REPLACEMENT / Resolution: 2.51 Å | ||||||
 Authors Authors | Niwa, H. / Sato, S. / Umehara, T. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: Acs Med.Chem.Lett. / Year: 2022 Journal: Acs Med.Chem.Lett. / Year: 2022Title: Structure-Activity Relationship and In Silico Evaluation of cis- and trans-PCPA-Derived Inhibitors of LSD1 and LSD2 Authors: Niwa, H. / Watanabe, C. / Sato, S. / Harada, T. / Watanabe, H. / Tabusa, R. / Fukasawa, S. / Shiobara, A. / Hashimoto, T. / Ohno, O. / Nakamura, K. / Tsuganezawa, K. / Tanaka, A. / Shirouzu, ...Authors: Niwa, H. / Watanabe, C. / Sato, S. / Harada, T. / Watanabe, H. / Tabusa, R. / Fukasawa, S. / Shiobara, A. / Hashimoto, T. / Ohno, O. / Nakamura, K. / Tsuganezawa, K. / Tanaka, A. / Shirouzu, M. / Honma, T. / Matsuno, K. / Umehara, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7w3l.cif.gz 7w3l.cif.gz | 278.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7w3l.ent.gz pdb7w3l.ent.gz | 223.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7w3l.json.gz 7w3l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w3/7w3l https://data.pdbj.org/pub/pdb/validation_reports/w3/7w3l ftp://data.pdbj.org/pub/pdb/validation_reports/w3/7w3l ftp://data.pdbj.org/pub/pdb/validation_reports/w3/7w3l | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7xe1C  7xe2C  7xe3C  6kgpS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 74333.039 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KDM1A, AOF2, KDM1, KIAA0601, LSD1 / Plasmid: PETDUET-1 / Production host: Homo sapiens (human) / Gene: KDM1A, AOF2, KDM1, KIAA0601, LSD1 / Plasmid: PETDUET-1 / Production host:  References: UniProt: O60341, [histone H3]-N6,N6-dimethyl-L-lysine4 FAD-dependent demethylase |
|---|
-Non-polymers , 5 types, 135 molecules 
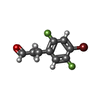







| #2: Chemical | ChemComp-FAD / | ||
|---|---|---|---|
| #3: Chemical | ChemComp-8A2 / | ||
| #4: Chemical | ChemComp-TLA / | ||
| #5: Chemical | ChemComp-GOL / #6: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.63 Å3/Da / Density % sol: 66.08 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 0.1M MES (pH 6.6), 0.2M diammonium tartrate, 0.0005M TCEP, 10-12% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06DA / Wavelength: 0.919 Å / Beamline: X06DA / Wavelength: 0.919 Å |
| Detector | Type: DECTRIS PILATUS 2M-F / Detector: PIXEL / Date: Mar 17, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.919 Å / Relative weight: 1 |
| Reflection | Resolution: 2.51→48.12 Å / Num. obs: 38005 / % possible obs: 99.9 % / Redundancy: 20 % / Biso Wilson estimate: 60.06 Å2 / Rsym value: 0.121 / Net I/σ(I): 20.4 |
| Reflection shell | Resolution: 2.51→2.61 Å / Redundancy: 21 % / Mean I/σ(I) obs: 1.8 / Rsym value: 2.347 / % possible all: 99.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6KGP Resolution: 2.51→48.12 Å / SU ML: 0.346 / Cross valid method: FREE R-VALUE / σ(F): 0.47 / Phase error: 27.207 Stereochemistry target values: GEOSTD + MONOMER LIBRARY + CDL V1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 74.15 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.51→48.12 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj










