[English] 日本語
 Yorodumi
Yorodumi- PDB-7un5: Structure of Type II Prion filaments from Gerstmann-Straussler-Sc... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7un5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Type II Prion filaments from Gerstmann-Straussler-Scheinker disease | |||||||||
 Components Components | Major prion protein | |||||||||
 Keywords Keywords | PROTEIN FIBRIL / Prion / PrP / GSS / filament / fibril / human brain derived / neurodegenerative | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of amyloid precursor protein catabolic process / regulation of glutamate receptor signaling pathway / lamin binding / aspartic-type endopeptidase inhibitor activity / positive regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / glycosaminoglycan binding / NCAM1 interactions / type 5 metabotropic glutamate receptor binding / ATP-dependent protein binding ...negative regulation of amyloid precursor protein catabolic process / regulation of glutamate receptor signaling pathway / lamin binding / aspartic-type endopeptidase inhibitor activity / positive regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / glycosaminoglycan binding / NCAM1 interactions / type 5 metabotropic glutamate receptor binding / ATP-dependent protein binding / negative regulation of interleukin-17 production / cupric ion binding / regulation of potassium ion transmembrane transport / negative regulation of protein processing / negative regulation of dendritic spine maintenance / dendritic spine maintenance / extrinsic component of membrane / negative regulation of calcineurin-NFAT signaling cascade / negative regulation of interleukin-2 production / negative regulation of T cell receptor signaling pathway / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of activated T cell proliferation / negative regulation of amyloid-beta formation / response to amyloid-beta / negative regulation of type II interferon production / cuprous ion binding / negative regulation of long-term synaptic potentiation / intracellular copper ion homeostasis / positive regulation of protein targeting to membrane / response to cadmium ion / long-term memory / inclusion body / neuron projection maintenance / positive regulation of calcium-mediated signaling / tubulin binding / molecular function activator activity / cellular response to copper ion / positive regulation of protein localization to plasma membrane / molecular condensate scaffold activity / protein homooligomerization / protein destabilization / cellular response to xenobiotic stimulus / cellular response to amyloid-beta / terminal bouton / positive regulation of neuron apoptotic process / signaling receptor activity / protein-folding chaperone binding / amyloid-beta binding / response to oxidative stress / protease binding / nuclear membrane / microtubule binding / molecular adaptor activity / transmembrane transporter binding / learning or memory / postsynapse / regulation of cell cycle / intracellular signal transduction / postsynaptic density / membrane raft / copper ion binding / external side of plasma membrane / intracellular membrane-bounded organelle / dendrite / negative regulation of apoptotic process / protein-containing complex binding / cell surface / negative regulation of transcription by RNA polymerase II / endoplasmic reticulum / Golgi apparatus / extracellular exosome / identical protein binding / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / helical reconstruction / cryo EM / Resolution: 3.13 Å | |||||||||
 Authors Authors | Ozcan, K.A. / Hoq, M.R. / Bharath, S.R. / Jiang, W. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Acta Neuropathol / Year: 2022 Journal: Acta Neuropathol / Year: 2022Title: Cryo-EM structures of prion protein filaments from Gerstmann-Sträussler-Scheinker disease. Authors: Grace I Hallinan / Kadir A Ozcan / Md Rejaul Hoq / Laura Cracco / Frank S Vago / Sakshibeedu R Bharath / Daoyi Li / Max Jacobsen / Emma H Doud / Amber L Mosley / Anllely Fernandez / Holly J ...Authors: Grace I Hallinan / Kadir A Ozcan / Md Rejaul Hoq / Laura Cracco / Frank S Vago / Sakshibeedu R Bharath / Daoyi Li / Max Jacobsen / Emma H Doud / Amber L Mosley / Anllely Fernandez / Holly J Garringer / Wen Jiang / Bernardino Ghetti / Ruben Vidal /  Abstract: Prion protein (PrP) aggregation and formation of PrP amyloid (APrP) are central events in the pathogenesis of prion diseases. In the dominantly inherited prion protein amyloidosis known as Gerstmann- ...Prion protein (PrP) aggregation and formation of PrP amyloid (APrP) are central events in the pathogenesis of prion diseases. In the dominantly inherited prion protein amyloidosis known as Gerstmann-Sträussler-Scheinker (GSS) disease, plaques made of PrP amyloid are present throughout the brain. The c.593t > c mutation in the prion protein gene (PRNP) results in a phenylalanine to serine amino acid substitution at PrP residue 198 (F198S) and causes the most severe amyloidosis among GSS variants. It has been shown that neurodegeneration in this disease is associated with the presence of extracellular APrP plaques and neuronal intracytoplasmic Tau inclusions, that have been shown to contain paired helical filaments identical to those found in Alzheimer disease. Using cryogenic electron microscopy (cryo-EM), we determined for the first time the structures of filaments of human APrP, isolated post-mortem from the brain of two symptomatic PRNP F198S mutation carriers. We report that in GSS (F198S) APrP filaments are composed of dimeric, trimeric and tetrameric left-handed protofilaments with their protomers sharing a common protein fold. The protomers in the cross-β spines consist of 62 amino acids and span from glycine 80 to phenylalanine 141, adopting a previously unseen spiral fold with a thicker outer layer and a thinner inner layer. Each protomer comprises nine short β-strands, with the β1 and β8 strands, as well as the β4 and β9 strands, forming a steric zipper. The data obtained by cryo-EM provide insights into the structural complexity of the PrP filament in a dominantly inherited human PrP amyloidosis. The novel findings highlight the urgency of extending our knowledge of the filaments' structures that may underlie distinct clinical and pathologic phenotypes of human neurodegenerative diseases. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7un5.cif.gz 7un5.cif.gz | 102.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7un5.ent.gz pdb7un5.ent.gz | 80.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7un5.json.gz 7un5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7un5_validation.pdf.gz 7un5_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7un5_full_validation.pdf.gz 7un5_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  7un5_validation.xml.gz 7un5_validation.xml.gz | 30.2 KB | Display | |
| Data in CIF |  7un5_validation.cif.gz 7un5_validation.cif.gz | 42.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/un/7un5 https://data.pdbj.org/pub/pdb/validation_reports/un/7un5 ftp://data.pdbj.org/pub/pdb/validation_reports/un/7un5 ftp://data.pdbj.org/pub/pdb/validation_reports/un/7un5 | HTTPS FTP |
-Related structure data
| Related structure data |  26613MC  7umqC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 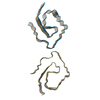
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 6219.043 Da / Num. of mol.: 10 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / Organ: Brain / Tissue: Cerebellum / References: UniProt: P04156 Homo sapiens (human) / Organ: Brain / Tissue: Cerebellum / References: UniProt: P04156 |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: FILAMENT / 3D reconstruction method: helical reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Type II Prion protein filament from Gerstmann-Straussler-Scheinker disease Type: TISSUE / Entity ID: all / Source: NATURAL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Brain / Tissue: Cerebellum Homo sapiens (human) / Organ: Brain / Tissue: Cerebellum | ||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||
| Buffer component |
| ||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||
| Specimen support | Details: Glow discharged using PELCO easiGlow at 15 mA for 10 seconds. Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||
| Vitrification | Instrument: GATAN CRYOPLUNGE 3 / Cryogen name: ETHANE / Humidity: 95 % / Chamber temperature: 293 K / Details: Plunge frozen in a BSL-2 hood |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 81000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 400 nm / Cs: 2.7 mm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 56.5 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 8600 |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Helical symmerty | Angular rotation/subunit: -0.94 ° / Axial rise/subunit: 4.83 Å / Axial symmetry: C1 | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.13 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 19341 / Symmetry type: HELICAL | ||||||||||||||||||||||||
| Atomic model building | Protocol: AB INITIO MODEL / Space: REAL | ||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 99.08 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj


