[English] 日本語
 Yorodumi
Yorodumi- PDB-7qox: Factor XI and Plasma Kallikrein apple domain structures reveals d... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7qox | ||||||
|---|---|---|---|---|---|---|---|
| Title | Factor XI and Plasma Kallikrein apple domain structures reveals different kininogen bound complexes | ||||||
 Components Components |
| ||||||
 Keywords Keywords | BLOOD CLOTTING / Plasma kallikrein (PK) / High molecular weight kininogen (HK) / Factor XII (FXII) / Factor XI (FXI) / Factor IX (FIX) / bradykinin (BK) | ||||||
| Function / homology |  Function and homology information Function and homology informationplasma kallikrein / Factor XII activation / Defective SERPING1 causes hereditary angioedema / positive regulation of fibrinolysis / negative regulation of cell adhesion / zymogen activation / plasminogen activation / Defective factor XII causes hereditary angioedema / Activation of Matrix Metalloproteinases / cysteine-type endopeptidase inhibitor activity ...plasma kallikrein / Factor XII activation / Defective SERPING1 causes hereditary angioedema / positive regulation of fibrinolysis / negative regulation of cell adhesion / zymogen activation / plasminogen activation / Defective factor XII causes hereditary angioedema / Activation of Matrix Metalloproteinases / cysteine-type endopeptidase inhibitor activity / negative regulation of blood coagulation / fibrinolysis / Intrinsic Pathway of Fibrin Clot Formation / negative regulation of proteolysis / Peptide ligand-binding receptors / platelet alpha granule lumen / Post-translational protein phosphorylation / hormone activity / vasodilation / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / blood coagulation / Platelet degranulation / heparin binding / : / positive regulation of cytosolic calcium ion concentration / blood microparticle / G alpha (i) signalling events / G alpha (q) signalling events / positive regulation of apoptotic process / inflammatory response / endoplasmic reticulum lumen / signaling receptor binding / serine-type endopeptidase activity / proteolysis / extracellular space / extracellular exosome / extracellular region / zinc ion binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.32 Å molecular replacement / Resolution: 2.32 Å | ||||||
 Authors Authors | Li, C. / Awital, B. / Wong, S. / Dreveny, I. / Meijers, J. / Emsley, J. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: J Thromb Haemost / Year: 2019 Journal: J Thromb Haemost / Year: 2019Title: Plasma kallikrein structure reveals apple domain disc rotated conformation compared to factor XI. Authors: Li, C. / Voos, K.M. / Pathak, M. / Hall, G. / McCrae, K.R. / Dreveny, I. / Li, R. / Emsley, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7qox.cif.gz 7qox.cif.gz | 174.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7qox.ent.gz pdb7qox.ent.gz | 134.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7qox.json.gz 7qox.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7qox_validation.pdf.gz 7qox_validation.pdf.gz | 3.9 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7qox_full_validation.pdf.gz 7qox_full_validation.pdf.gz | 3.9 MB | Display | |
| Data in XML |  7qox_validation.xml.gz 7qox_validation.xml.gz | 34.2 KB | Display | |
| Data in CIF |  7qox_validation.cif.gz 7qox_validation.cif.gz | 48.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qo/7qox https://data.pdbj.org/pub/pdb/validation_reports/qo/7qox ftp://data.pdbj.org/pub/pdb/validation_reports/qo/7qox ftp://data.pdbj.org/pub/pdb/validation_reports/qo/7qox | HTTPS FTP |
-Related structure data
| Related structure data |  6i44C  6i58SC  7qotC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
|
- Components
Components
-Protein / Protein/peptide / Sugars , 3 types, 6 molecules ABDC

| #1: Protein | Mass: 41134.977 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KLKB1, KLK3 / Production host: Homo sapiens (human) / Gene: KLKB1, KLK3 / Production host:  #2: Protein/peptide | Mass: 3263.391 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: KNG1, BDK, KNG / Production host: Homo sapiens (human) / Gene: KNG1, BDK, KNG / Production host:  #7: Sugar | |
|---|
-Non-polymers , 6 types, 346 molecules 



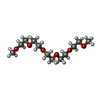






| #3: Chemical | ChemComp-GOL / #4: Chemical | ChemComp-PEG / #5: Chemical | #6: Chemical | #8: Chemical | ChemComp-PG6 / | #9: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.73 % |
|---|---|
| Crystal grow | Temperature: 288 K / Method: evaporation Details: Morpheus screen H9 condition: 0.1M amino acids, 0.1M buffer system 3 pH8.5, 30% precipitant mix 1. |
-Data collection
| Diffraction | Mean temperature: 93 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.976 Å / Beamline: I03 / Wavelength: 0.976 Å |
| Detector | Type: DECTRIS EIGER2 XE 16M / Detector: PIXEL / Date: May 25, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976 Å / Relative weight: 1 |
| Reflection | Resolution: 2.32→29.64 Å / Num. obs: 38760 / % possible obs: 99.4 % / Redundancy: 3.4 % / Biso Wilson estimate: 29.46 Å2 / CC1/2: 0.987 / Net I/σ(I): 7.6 |
| Reflection shell | Resolution: 2.32→2.4 Å / Num. unique obs: 9002 / CC1/2: 0.597 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6I58 Resolution: 2.32→29.64 Å / SU ML: 0.32 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 25.15 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 99.07 Å2 / Biso mean: 33.7441 Å2 / Biso min: 14.06 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.32→29.64 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 14
|
 Movie
Movie Controller
Controller


 PDBj
PDBj














