[English] 日本語
 Yorodumi
Yorodumi- PDB-7qb5: Coxsackievirus A24v (CVA24v) in complex with a dimeric C2-C9-link... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7qb5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Coxsackievirus A24v (CVA24v) in complex with a dimeric C2-C9-linked sialic acid inhibitor | ||||||
 Components Components | (Capsid protein ...) x 4 | ||||||
 Keywords Keywords | VIRUS / Coxsackievirus A24v / CVA24v / sialic acid based inhibitor | ||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / channel activity ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | ||||||
| Biological species |  Coxsackievirus A24 Coxsackievirus A24 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.728 Å MOLECULAR REPLACEMENT / Resolution: 1.728 Å | ||||||
 Authors Authors | Zocher, G. / Stehle, T. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Rsc Adv / Year: 2022 Journal: Rsc Adv / Year: 2022Title: Exploring divalent conjugates of 5- N -acetyl-neuraminic acid as inhibitors of coxsackievirus A24 variant (CVA24v) transduction. Authors: Johansson, E. / Caraballo, R. / Zocher, G. / Mistry, N. / Arnberg, N. / Stehle, T. / Elofsson, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7qb5.cif.gz 7qb5.cif.gz | 212.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7qb5.ent.gz pdb7qb5.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7qb5.json.gz 7qb5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qb/7qb5 https://data.pdbj.org/pub/pdb/validation_reports/qb/7qb5 ftp://data.pdbj.org/pub/pdb/validation_reports/qb/7qb5 ftp://data.pdbj.org/pub/pdb/validation_reports/qb/7qb5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4q4wS S: Starting model for refinement |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Capsid protein ... , 4 types, 4 molecules 111222333444
| #1: Protein | Mass: 34378.371 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Coxsackievirus A24 / Strain: A24v / Cell line (production host): corneal cells / Production host: Coxsackievirus A24 / Strain: A24v / Cell line (production host): corneal cells / Production host:  Homo sapiens (human) / References: UniProt: V9VEF3 Homo sapiens (human) / References: UniProt: V9VEF3 |
|---|---|
| #2: Protein | Mass: 29817.412 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Coxsackievirus A24 / Strain: A24v / Cell line: corneal cells / Cell line (production host): corneal cells / Production host: Coxsackievirus A24 / Strain: A24v / Cell line: corneal cells / Cell line (production host): corneal cells / Production host:  Homo sapiens (human) / References: UniProt: V9VEF3 Homo sapiens (human) / References: UniProt: V9VEF3 |
| #3: Protein | Mass: 26637.746 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Coxsackievirus A24 / Strain: A24v / Cell line (production host): corneal cells / Production host: Coxsackievirus A24 / Strain: A24v / Cell line (production host): corneal cells / Production host:  Homo sapiens (human) / References: UniProt: V9VEF3 Homo sapiens (human) / References: UniProt: V9VEF3 |
| #4: Protein | Mass: 7319.045 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Coxsackievirus A24 / Strain: A24v / Variant: variant / Cell line (production host): corneal cells / Production host: Coxsackievirus A24 / Strain: A24v / Variant: variant / Cell line (production host): corneal cells / Production host:  Homo sapiens (human) / References: UniProt: V9VEF3 Homo sapiens (human) / References: UniProt: V9VEF3 |
-Sugars , 1 types, 1 molecules 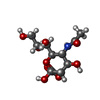
| #5: Sugar | ChemComp-SIA / |
|---|
-Non-polymers , 4 types, 820 molecules 






| #6: Chemical | ChemComp-HEZ / | ||||
|---|---|---|---|---|---|
| #7: Chemical | ChemComp-CA / #8: Chemical | ChemComp-CL / #9: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 200 mM Magnesium chloride, 3.4 M 1,6-Hexanediol, 100 mM HEPES pH 7.5 |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9762 Å / Beamline: I03 / Wavelength: 0.9762 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Apr 26, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9762 Å / Relative weight: 1 |
| Reflection | Resolution: 1.728→50 Å / Num. obs: 6338512 / % possible obs: 91.4 % / Redundancy: 6.6 % / CC1/2: 0.992 / Rrim(I) all: 0.158 / Net I/σ(I): 6.5 |
| Reflection shell | Resolution: 1.73→1.83 Å / Redundancy: 3.1 % / Mean I/σ(I) obs: 1.4 / Num. unique obs: 973400 / CC1/2: 0.604 / Rrim(I) all: 0.79 / % possible all: 93.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4Q4W Resolution: 1.728→49.922 Å / Cor.coef. Fo:Fc: 0.962 / SU B: 1.937 / SU ML: 0.057 / Cross valid method: NONE / ESU R: 0.019 Details: Hydrogens have been added in their riding positions
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL PLUS MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.874 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.728→49.922 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj






