+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 7nhh | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Crystal structure of the human METTL3-METTL14 complex with compound UOZ002 | ||||||
 要素 要素 | (N6-adenosine-methyltransferase ...) x 2 | ||||||
 キーワード キーワード | TRANSFERASE / METTL3 / METTL14 / M6A / Inhibitor / Complex | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of hematopoietic progenitor cell differentiation / positive regulation of cap-independent translational initiation / mRNA m6A methyltransferase / mRNA m(6)A methyltransferase activity / RNA N6-methyladenosine methyltransferase complex / RNA methylation / endothelial to hematopoietic transition / regulation of meiotic cell cycle / RNA methyltransferase activity / primary miRNA processing ...negative regulation of hematopoietic progenitor cell differentiation / positive regulation of cap-independent translational initiation / mRNA m6A methyltransferase / mRNA m(6)A methyltransferase activity / RNA N6-methyladenosine methyltransferase complex / RNA methylation / endothelial to hematopoietic transition / regulation of meiotic cell cycle / RNA methyltransferase activity / primary miRNA processing / forebrain radial glial cell differentiation / dosage compensation by inactivation of X chromosome / oxidoreductase complex / S-adenosyl-L-methionine binding / gliogenesis / mRNA stabilization / regulation of hematopoietic stem cell differentiation / mRNA modification / regulation of neuron differentiation / regulation of T cell differentiation / negative regulation of type I interferon-mediated signaling pathway / oogenesis / stem cell population maintenance / mRNA destabilization / Processing of Capped Intron-Containing Pre-mRNA / negative regulation of Notch signaling pathway / positive regulation of translation / response to nutrient levels / circadian rhythm / mRNA splicing, via spliceosome / mRNA processing / cellular response to UV / spermatogenesis / nuclear speck / nuclear body / protein heterodimerization activity / innate immune response / mRNA binding / DNA damage response / Golgi apparatus / nucleoplasm / nucleus / cytosol 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 |  X線回折 / X線回折 /  シンクロトロン / シンクロトロン /  分子置換 / 解像度: 2.1 Å 分子置換 / 解像度: 2.1 Å | ||||||
 データ登録者 データ登録者 | Bedi, R.K. / Huang, D. / Caflisch, A. | ||||||
| 資金援助 |  スイス, 1件 スイス, 1件
| ||||||
 引用 引用 |  ジャーナル: Acs Bio Med Chem Au / 年: 2023 ジャーナル: Acs Bio Med Chem Au / 年: 2023タイトル: Structure-Based Design of Inhibitors of the m6A-RNA Writer Enzyme METTL3 著者: Bedi, R.K. / Huang, D. / Li, Y. / Caflisch, A. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  7nhh.cif.gz 7nhh.cif.gz | 130.9 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb7nhh.ent.gz pdb7nhh.ent.gz | 79 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  7nhh.json.gz 7nhh.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  7nhh_validation.pdf.gz 7nhh_validation.pdf.gz | 738.6 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  7nhh_full_validation.pdf.gz 7nhh_full_validation.pdf.gz | 742 KB | 表示 | |
| XML形式データ |  7nhh_validation.xml.gz 7nhh_validation.xml.gz | 19.6 KB | 表示 | |
| CIF形式データ |  7nhh_validation.cif.gz 7nhh_validation.cif.gz | 27.9 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/nh/7nhh https://data.pdbj.org/pub/pdb/validation_reports/nh/7nhh ftp://data.pdbj.org/pub/pdb/validation_reports/nh/7nhh ftp://data.pdbj.org/pub/pdb/validation_reports/nh/7nhh | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7nhgC  7nhiC  7nhjC  7nhvC  7ni7C  7ni8C  7ni9C  7niaC  7nidC  7oedC  7oeeC  7oefC  7oegC  7oehC  7oeiC  7oejC  7oekC  7oelC  7oemC  7oqlC  7oqoC  7oqpC  5l6dS S: 精密化の開始モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ |
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| 単位格子 |
|
- 要素
要素
-N6-adenosine-methyltransferase ... , 2種, 2分子 AB
| #1: タンパク質 | 分子量: 28144.080 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: METTL3, MTA70 Homo sapiens (ヒト) / 遺伝子: METTL3, MTA70発現宿主:  参照: UniProt: Q86U44, mRNA m6A methyltransferase |
|---|---|
| #2: タンパク質 | 分子量: 33621.246 Da / 分子数: 1 / 由来タイプ: 組換発現 / 由来: (組換発現)  Homo sapiens (ヒト) / 遺伝子: METTL14, KIAA1627 Homo sapiens (ヒト) / 遺伝子: METTL14, KIAA1627発現宿主:  参照: UniProt: Q9HCE5 |
-非ポリマー , 4種, 174分子 
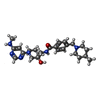





| #3: 化合物 | ChemComp-MG / #4: 化合物 | ChemComp-UDK / ( | #5: 化合物 | ChemComp-ACT / | #6: 水 | ChemComp-HOH / | |
|---|
-詳細
| 研究の焦点であるリガンドがあるか | Y |
|---|---|
| Has protein modification | Y |
-実験情報
-実験
| 実験 | 手法:  X線回折 / 使用した結晶の数: 1 X線回折 / 使用した結晶の数: 1 |
|---|
- 試料調製
試料調製
| 結晶 | マシュー密度: 2.17 Å3/Da / 溶媒含有率: 43.23 % |
|---|---|
| 結晶化 | 温度: 295 K / 手法: 蒸気拡散法, ハンギングドロップ法 / 詳細: 20% PEG 3350, 400mM Mg acetate |
-データ収集
| 回折 | 平均測定温度: 100 K / Serial crystal experiment: N |
|---|---|
| 放射光源 | 由来:  シンクロトロン / サイト: シンクロトロン / サイト:  SLS SLS  / ビームライン: X06DA / 波長: 1 Å / ビームライン: X06DA / 波長: 1 Å |
| 検出器 | タイプ: DECTRIS PILATUS 2M-F / 検出器: PIXEL / 日付: 2018年11月15日 |
| 放射 | プロトコル: SINGLE WAVELENGTH / 単色(M)・ラウエ(L): M / 散乱光タイプ: x-ray |
| 放射波長 | 波長: 1 Å / 相対比: 1 |
| 反射 | 解像度: 2.1→45.2 Å / Num. obs: 32430 / % possible obs: 99.8 % / 冗長度: 6.5 % / Biso Wilson estimate: 44.02 Å2 / CC1/2: 0.999 / Net I/σ(I): 17.56 |
| 反射 シェル | 解像度: 2.1→2.23 Å / Num. unique obs: 5165 / CC1/2: 0.497 |
- 解析
解析
| ソフトウェア |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 精密化 | 構造決定の手法:  分子置換 分子置換開始モデル: 5L6D 解像度: 2.1→44.67 Å / SU ML: 0.2698 / 交差検証法: FREE R-VALUE / σ(F): 1.35 / 位相誤差: 26.4807 立体化学のターゲット値: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 溶媒の処理 | 減衰半径: 0.9 Å / VDWプローブ半径: 1.11 Å / 溶媒モデル: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子変位パラメータ | Biso mean: 49.19 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 精密化ステップ | サイクル: LAST / 解像度: 2.1→44.67 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 拘束条件 |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS精密化 シェル |
|
 ムービー
ムービー コントローラー
コントローラー















 PDBj
PDBj


