Entry Database : PDB / ID : 7bnuTitle VDR complex with BXL-62 Nuclear receptor coactivator 1 Vitamin D3 receptor A Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Danio rerio (zebrafish)Homo sapiens (human)Method / / / Resolution : 2.4 Å Authors Rochel, N. / Belorusova, A.Y. Funding support Organization Grant number Country Agence Nationale de la Recherche (ANR) ANR-13-BSV8-0024-01
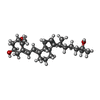
Journal : Int J Mol Sci / Year : 2022Title : Vitamin D Analogs Bearing C-20 Modifications Stabilize the Agonistic Conformation of Non-Responsive Vitamin D Receptor Variants.Authors : Belorusova, A.Y. / Rovito, D. / Chebaro, Y. / Doms, S. / Verlinden, L. / Verstuyf, A. / Metzger, D. / Rochel, N. / Laverny, G. History Deposition Jan 22, 2021 Deposition site / Processing site Revision 1.0 Mar 2, 2022 Provider / Type Revision 1.1 Sep 21, 2022 Group / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year Revision 1.2 Jan 31, 2024 Group / Refinement descriptionCategory / chem_comp_bond / pdbx_initial_refinement_model
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å
MOLECULAR REPLACEMENT / Resolution: 2.4 Å  Authors
Authors France, 1items
France, 1items  Citation
Citation Journal: Int J Mol Sci / Year: 2022
Journal: Int J Mol Sci / Year: 2022 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 7bnu.cif.gz
7bnu.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb7bnu.ent.gz
pdb7bnu.ent.gz PDB format
PDB format 7bnu.json.gz
7bnu.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/bn/7bnu
https://data.pdbj.org/pub/pdb/validation_reports/bn/7bnu ftp://data.pdbj.org/pub/pdb/validation_reports/bn/7bnu
ftp://data.pdbj.org/pub/pdb/validation_reports/bn/7bnu

 Links
Links Assembly
Assembly
 Components
Components

 Homo sapiens (human) / References: UniProt: Q15788, histone acetyltransferase
Homo sapiens (human) / References: UniProt: Q15788, histone acetyltransferase X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID23-2 / Wavelength: 1 Å
/ Beamline: ID23-2 / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller























 PDBj
PDBj