+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6qas | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of ULK1 in complexed with PF-03814735 | ||||||
 Components Components | Serine/threonine-protein kinase ULK1 | ||||||
 Keywords Keywords | TRANSFERASE / ULK1 / autophagy / ATG1 / kinase / inhibitor complex / Structural Genomics / Structural Genomics Consortium / SGC | ||||||
| Function / homology |  Function and homology information Function and homology informationneuron projection regeneration / omegasome membrane / negative regulation of collateral sprouting / Atg1/ULK1 kinase complex / positive regulation of autophagosome assembly / phagophore assembly site membrane / piecemeal microautophagy of the nucleus / RAB GEFs exchange GTP for GDP on RABs / regulation of tumor necrosis factor-mediated signaling pathway / phagophore assembly site ...neuron projection regeneration / omegasome membrane / negative regulation of collateral sprouting / Atg1/ULK1 kinase complex / positive regulation of autophagosome assembly / phagophore assembly site membrane / piecemeal microautophagy of the nucleus / RAB GEFs exchange GTP for GDP on RABs / regulation of tumor necrosis factor-mediated signaling pathway / phagophore assembly site / axon extension / TBC/RABGAPs / reticulophagy / response to starvation / cellular response to stress / Macroautophagy / Receptor Mediated Mitophagy / autophagosome membrane / autophagosome assembly / regulation of macroautophagy / cellular response to nutrient levels / negative regulation of protein-containing complex assembly / mitophagy / positive regulation of autophagy / autophagosome / macroautophagy / Regulation of TNFR1 signaling / recycling endosome / peptidyl-serine phosphorylation / autophagy / small GTPase binding / neuron projection development / intracellular protein localization / protein autophosphorylation / GTPase binding / mitochondrial outer membrane / protein phosphorylation / non-specific serine/threonine protein kinase / regulation of autophagy / axon / negative regulation of cell population proliferation / protein serine kinase activity / protein serine/threonine kinase activity / endoplasmic reticulum membrane / protein-containing complex binding / signal transduction / ATP binding / identical protein binding / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.75 Å MOLECULAR REPLACEMENT / Resolution: 1.75 Å | ||||||
 Authors Authors | Chaikuad, A. / Arrowsmith, C.H. / Edwards, A.M. / Bountra, C. / Knapp, S. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: Biochem.J. / Year: 2019 Journal: Biochem.J. / Year: 2019Title: Conservation of structure, function and inhibitor binding in UNC-51-like kinase 1 and 2 (ULK1/2). Authors: Chaikuad, A. / Koschade, S.E. / Stolz, A. / Zivkovic, K. / Pohl, C. / Shaid, S. / Ren, H. / Lambert, L.J. / Cosford, N.D.P. / Brandts, C.H. / Knapp, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6qas.cif.gz 6qas.cif.gz | 247.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6qas.ent.gz pdb6qas.ent.gz | 199.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6qas.json.gz 6qas.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qa/6qas https://data.pdbj.org/pub/pdb/validation_reports/qa/6qas ftp://data.pdbj.org/pub/pdb/validation_reports/qa/6qas ftp://data.pdbj.org/pub/pdb/validation_reports/qa/6qas | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6qatC  6qauC  6qavC  4wnoS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||
| 2 | 
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Ens-ID: 1 / Beg auth comp-ID: THR / Beg label comp-ID: THR / End auth comp-ID: ASP / End label comp-ID: ASP / Refine code: _ / Auth seq-ID: 8 - 279 / Label seq-ID: 12 - 283
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 32400.238 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ULK1, KIAA0722 / Plasmid: pNIC28-Bsa4 / Production host: Homo sapiens (human) / Gene: ULK1, KIAA0722 / Plasmid: pNIC28-Bsa4 / Production host:  References: UniProt: O75385, non-specific serine/threonine protein kinase |
|---|
-Non-polymers , 6 types, 419 molecules 



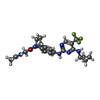






| #2: Chemical | ChemComp-SO4 / #3: Chemical | #4: Chemical | ChemComp-GOL / #5: Chemical | ChemComp-EDO / #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.36 Å3/Da / Density % sol: 47.96 % |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, sitting drop / pH: 5.6 / Details: 2.8 M Ammonium sulfate, 0.1 M citrate, pH 5.6 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 1 Å / Beamline: X06SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Sep 21, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→47.47 Å / Num. obs: 63838 / % possible obs: 100 % / Redundancy: 9.2 % / Biso Wilson estimate: 23 Å2 / CC1/2: 0.997 / Rmerge(I) obs: 0.079 / Net I/σ(I): 14.4 |
| Reflection shell | Resolution: 1.75→1.81 Å / Redundancy: 9.4 % / Rmerge(I) obs: 0.777 / Mean I/σ(I) obs: 2.7 / Num. unique obs: 6154 / CC1/2: 0.806 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4WNO Resolution: 1.75→47.47 Å / Cor.coef. Fo:Fc: 0.966 / Cor.coef. Fo:Fc free: 0.958 / SU B: 3.975 / SU ML: 0.065 / Cross valid method: THROUGHOUT / ESU R: 0.108 / ESU R Free: 0.102 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 31.23 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.206 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.75→47.47 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj











