[English] 日本語
 Yorodumi
Yorodumi- PDB-6gad: BACTERIORHODOPSIN, 530 FS STATE, REAL-SPACE REFINED AGAINST 15% E... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6gad | ||||||
|---|---|---|---|---|---|---|---|
| Title | BACTERIORHODOPSIN, 530 FS STATE, REAL-SPACE REFINED AGAINST 15% EXTRAPOLATED STRUCTURE FACTORS | ||||||
 Components Components | Bacteriorhodopsin | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / proton pump / time-resolved crystallography / free-electron laser | ||||||
| Function / homology |  Function and homology information Function and homology informationlight-driven active monoatomic ion transmembrane transporter activity / monoatomic ion channel activity / photoreceptor activity / phototransduction / proton transmembrane transport / plasma membrane Similarity search - Function | ||||||
| Biological species |  Halobacterium salinarum (Halophile) Halobacterium salinarum (Halophile) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / Resolution: 1.8 Å FREE ELECTRON LASER / Resolution: 1.8 Å | ||||||
 Authors Authors | Nass Kovacs, G. / Colletier, J.-P. / Gruenbein, M.L. / Stensitzki, T. / Batyuk, A. / Carbajo, S. / Doak, R.B. / Ehrenberg, D. / Foucar, L. / Gasper, R. ...Nass Kovacs, G. / Colletier, J.-P. / Gruenbein, M.L. / Stensitzki, T. / Batyuk, A. / Carbajo, S. / Doak, R.B. / Ehrenberg, D. / Foucar, L. / Gasper, R. / Gorel, A. / Hilpert, M. / Kloos, M. / Koglin, J. / Reinstein, J. / Roome, C.M. / Schlesinger, R. / Seaberg, M. / Shoeman, R.L. / Stricker, M. / Boutet, S. / Haacke, S. / Heberle, J. / Domratcheva, T. / Schlichting, I. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Three-dimensional view of ultrafast dynamics in photoexcited bacteriorhodopsin. Authors: Nass Kovacs, G. / Colletier, J.P. / Grunbein, M.L. / Yang, Y. / Stensitzki, T. / Batyuk, A. / Carbajo, S. / Doak, R.B. / Ehrenberg, D. / Foucar, L. / Gasper, R. / Gorel, A. / Hilpert, M. / ...Authors: Nass Kovacs, G. / Colletier, J.P. / Grunbein, M.L. / Yang, Y. / Stensitzki, T. / Batyuk, A. / Carbajo, S. / Doak, R.B. / Ehrenberg, D. / Foucar, L. / Gasper, R. / Gorel, A. / Hilpert, M. / Kloos, M. / Koglin, J.E. / Reinstein, J. / Roome, C.M. / Schlesinger, R. / Seaberg, M. / Shoeman, R.L. / Stricker, M. / Boutet, S. / Haacke, S. / Heberle, J. / Heyne, K. / Domratcheva, T. / Barends, T.R.M. / Schlichting, I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6gad.cif.gz 6gad.cif.gz | 102.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6gad.ent.gz pdb6gad.ent.gz | 78.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6gad.json.gz 6gad.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ga/6gad https://data.pdbj.org/pub/pdb/validation_reports/ga/6gad ftp://data.pdbj.org/pub/pdb/validation_reports/ga/6gad ftp://data.pdbj.org/pub/pdb/validation_reports/ga/6gad | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6ga1C  6ga2C  6ga3C  6ga4C  6ga5C  6ga6C  6ga7C  6ga8C  6ga9C  6gaaC  6gabC  6gacC  6gaeC  6gafC  6gagC  6gahC  6gaiC  6rmkC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 26929.500 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Halobacterium salinarum (strain ATCC 700922 / JCM 11081 / NRC-1) (Halophile) Halobacterium salinarum (strain ATCC 700922 / JCM 11081 / NRC-1) (Halophile)Strain: ATCC 700922 / JCM 11081 / NRC-1 / Gene: bop, VNG_1467G / Production host:  Halobacterium salinarum (Halophile) / References: UniProt: P02945 Halobacterium salinarum (Halophile) / References: UniProt: P02945 |
|---|
-Non-polymers , 11 types, 53 molecules 
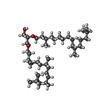


















| #2: Chemical | ChemComp-RET / | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #3: Chemical | | #4: Chemical | ChemComp-TRD / | #5: Chemical | ChemComp-D10 / | #6: Chemical | ChemComp-HP6 / | #7: Chemical | #8: Chemical | ChemComp-MYS / | #9: Chemical | #10: Chemical | ChemComp-DD9 / | #11: Chemical | ChemComp-C14 / | #12: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.28 Å3/Da / Density % sol: 46.15 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: lipidic cubic phase / pH: 5.8 / Details: 32% (w/v) PEG 2000, 0.1 M K2HPO4 /NaH2PO4 |
-Data collection
| Diffraction | Mean temperature: 293 K / Serial crystal experiment: Y |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: FREE ELECTRON LASER / Site:  SLAC LCLS SLAC LCLS  / Beamline: CXI / Wavelength: 1.26 Å / Beamline: CXI / Wavelength: 1.26 Å |
| Detector | Type: CS-PAD CXI-2 / Detector: PIXEL / Date: Jul 25, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.26 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→20 Å / Num. obs: 22397 / % possible obs: 100 % / Redundancy: 137 % / R split: 0.12 / Net I/σ(I): 6 |
| Reflection shell | Resolution: 1.8→1.9 Å / R split: 0.79 |
- Processing
Processing
| Software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.8→20 Å / Cross valid method: NONE Details: A MOLECULE IS THE DARK STATE B MOLECULE WAS REAL-SPACE REFINED AGAINST MAP CALCULATED FROM EXTRAPOLATED STRUCTURE FACTORS (AT 15% OCCUPANCY) MODEL/MAP FIT (CCmask)=0.8374
| ||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→20 Å
|
 Movie
Movie Controller
Controller












 PDBj
PDBj

















