[English] 日本語
 Yorodumi
Yorodumi- PDB-6e7t: Heterodimer of the GluN1b-GluN2B NMDA receptor amino-terminal dom... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6e7t | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Heterodimer of the GluN1b-GluN2B NMDA receptor amino-terminal domains bound to allosteric inhibitor 93-6 | |||||||||
 Components Components | (Glutamate receptor ionotropic, NMDA ...) x 2 | |||||||||
 Keywords Keywords | TRANSPORT PROTEIN / NMDA Receptor / Ion channel / Allosteric modulation / Extracellular domain | |||||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to curcumin / cellular response to corticosterone stimulus / cellular response to magnesium starvation / sensory organ development / EPHB-mediated forward signaling / regulation of cAMP/PKA signal transduction / Assembly and cell surface presentation of NMDA receptors / response to hydrogen sulfide / auditory behavior / sensitization ...cellular response to curcumin / cellular response to corticosterone stimulus / cellular response to magnesium starvation / sensory organ development / EPHB-mediated forward signaling / regulation of cAMP/PKA signal transduction / Assembly and cell surface presentation of NMDA receptors / response to hydrogen sulfide / auditory behavior / sensitization / response to other organism / dendritic branch / fear response / response to methylmercury / apical dendrite / regulation of ARF protein signal transduction / response to manganese ion / response to carbohydrate / suckling behavior / positive regulation of inhibitory postsynaptic potential / interleukin-1 receptor binding / cellular response to dsRNA / cellular response to lipid / response to growth hormone / negative regulation of dendritic spine maintenance / RAF/MAP kinase cascade / heterocyclic compound binding / response to amine / positive regulation of glutamate secretion / Synaptic adhesion-like molecules / regulation of monoatomic cation transmembrane transport / NMDA glutamate receptor activity / response to glycoside / NMDA selective glutamate receptor complex / glutamate binding / ligand-gated sodium channel activity / response to zinc ion / calcium ion transmembrane import into cytosol / protein heterotetramerization / glycine binding / small molecule binding / receptor clustering / startle response / parallel fiber to Purkinje cell synapse / behavioral response to pain / monoatomic cation transmembrane transport / regulation of MAPK cascade / response to magnesium ion / regulation of postsynaptic membrane potential / response to electrical stimulus / action potential / extracellularly glutamate-gated ion channel activity / associative learning / regulation of neuronal synaptic plasticity / Unblocking of NMDA receptors, glutamate binding and activation / monoatomic cation transport / glutamate receptor binding / response to mechanical stimulus / detection of mechanical stimulus involved in sensory perception of pain / long-term memory / neuron development / multicellular organismal response to stress / positive regulation of synaptic transmission, glutamatergic / postsynaptic density, intracellular component / behavioral fear response / response to fungicide / monoatomic cation channel activity / synaptic cleft / glutamate-gated receptor activity / cellular response to manganese ion / regulation of long-term synaptic depression / D2 dopamine receptor binding / response to cytokine / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / cell adhesion molecule binding / ionotropic glutamate receptor binding / ionotropic glutamate receptor signaling pathway / cellular response to forskolin / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / protein tyrosine kinase binding / synaptic membrane / sodium ion transmembrane transport / positive regulation of excitatory postsynaptic potential / response to amphetamine / hippocampal mossy fiber to CA3 synapse / learning / response to nicotine / response to cocaine / hippocampus development / synaptic transmission, glutamatergic / excitatory postsynaptic potential / regulation of membrane potential / cellular response to amino acid stimulus / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / response to calcium ion / regulation of long-term neuronal synaptic plasticity / beta-catenin binding / regulation of synaptic plasticity / cerebral cortex development Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.31 Å MOLECULAR REPLACEMENT / Resolution: 2.31 Å | |||||||||
 Authors Authors | Regan, M.C. / Furukawa, H. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Structural elements of a pH-sensitive inhibitor binding site in NMDA receptors Authors: Regan, M.C. / Furukawa, H. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6e7t.cif.gz 6e7t.cif.gz | 568 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6e7t.ent.gz pdb6e7t.ent.gz | 464.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6e7t.json.gz 6e7t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e7/6e7t https://data.pdbj.org/pub/pdb/validation_reports/e7/6e7t ftp://data.pdbj.org/pub/pdb/validation_reports/e7/6e7t ftp://data.pdbj.org/pub/pdb/validation_reports/e7/6e7t | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6e7rC  6e7sC  6e7uC  6e7vC  6e7wC  6e7xC  3qelS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Refine code: 1
NCS ensembles :
NCS oper:
|
- Components
Components
-Glutamate receptor ionotropic, NMDA ... , 2 types, 4 molecules ACBD
| #1: Protein | Mass: 43162.270 Da / Num. of mol.: 2 / Fragment: Extracellular residues 23-407 / Mutation: N61Q, N371Q Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Trichoplusia ni (cabbage looper) / References: UniProt: A0A1L8F5J9 Trichoplusia ni (cabbage looper) / References: UniProt: A0A1L8F5J9#2: Protein | Mass: 41280.820 Da / Num. of mol.: 2 / Fragment: Extracellular residues 32-394 / Mutation: N348D Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Trichoplusia ni (cabbage looper) / References: UniProt: Q00960 Trichoplusia ni (cabbage looper) / References: UniProt: Q00960 |
|---|
-Sugars , 2 types, 7 molecules 
| #3: Polysaccharide | alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2- ...alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|---|
| #4: Sugar | ChemComp-NAG / |
-Non-polymers , 4 types, 332 molecules 

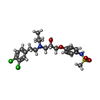




| #5: Chemical | | #6: Chemical | ChemComp-CL / #7: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.27 Å3/Da / Density % sol: 62.4 % |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 3.0-3.5 M sodium formate, 0.1 M HEPES, 35 mM sodium chloride, 7 mM Tris-HCl, 50 uM Ifenprodil |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS-II NSLS-II  / Beamline: 17-ID-2 / Wavelength: 0.91 Å / Beamline: 17-ID-2 / Wavelength: 0.91 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Feb 2, 2018 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.91 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.31→50 Å / Num. obs: 83106 / % possible obs: 91 % / Redundancy: 3.6 % / Rmerge(I) obs: 0.092 / Rpim(I) all: 0.054 / Rrim(I) all: 0.107 / Χ2: 0.923 / Net I/σ(I): 7.7 / Num. measured all: 299032 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3QEL Resolution: 2.31→25 Å / Cor.coef. Fo:Fc: 0.942 / Cor.coef. Fo:Fc free: 0.921 / SU B: 15.019 / SU ML: 0.173 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.351 / ESU R Free: 0.235 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 146.95 Å2 / Biso mean: 42.339 Å2 / Biso min: 14.13 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.31→25 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Dom-ID: 1 / Refine-ID: X-RAY DIFFRACTION / Type: TIGHT THERMAL / Weight position: 0.5
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.311→2.37 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







